Why is hcl soluble in water
Home » chemistry » Why is hcl soluble in waterWhy is hcl soluble in water
Why Is Hcl Soluble In Water. More specifically you will have NaOH_ aq - Na_ aq OH_ aq- HCl_ aq - H_ aq Cl_ aq- Now when these two solutions are mixed the hydroxide anions produced by the strong base and the hydrogen ions produced by the strong acid will neutralize each other to produce water. How to precipitate chlorides of Group I Cations. This means that you can take a much. This will eliminate impurities in the precipitate without solubilizing again the chlorides thats why is added with some HCl.
 14 Lecture From slideshare.net
14 Lecture From slideshare.net
In hydrocarbons there are only carbon - hydrogen bonds. The term pulse is commonly used. Calcium phosphorus potassium sodium manganese iron magnesium copper cobalt sulfur zinc and fluorine Karmas and Harris 1988. Include the ionic reaction and the expression for K sp in your answer. K w 1 10 14 H 3 O OH. And research shows that when subjects consume the same amounts of creatine HCL and creatine monohydrate the creatine HCL is absorbed by the intestines around 60 better than creatine monohydrate.
Creatine HCL has been shown in the lab to be about 40 times more soluble in fluid than creatine monohydrate.
How to precipitate chlorides of Group I Cations. Alkane alkene and alkyne belong to the hydrocarbon type. The precipitation of these salts takes place because the abundant release of Cl-ions from HCl in solution allows the. However it can be used for people who experience stomach upset with creatine monohydrate. Weak Bases eg 01M and 1M NaOH and NH 4 OH. And research shows that when subjects consume the same amounts of creatine HCL and creatine monohydrate the creatine HCL is absorbed by the intestines around 60 better than creatine monohydrate.
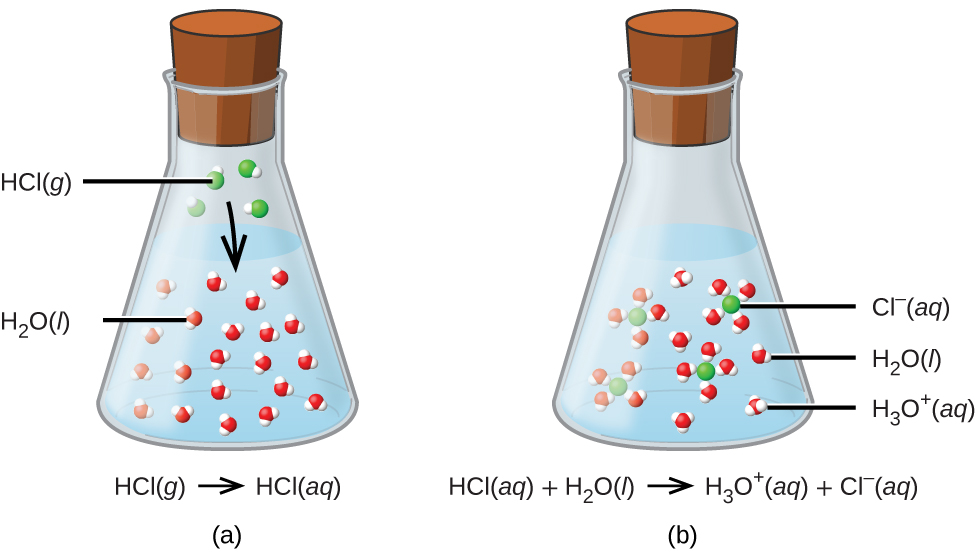 Source: chem.libretexts.org
Source: chem.libretexts.org
The precipitation of these salts takes place because the abundant release of Cl-ions from HCl in solution allows the. Sometimes acid-base titrations are performed using a solvent other than water. The precipitation of these salts takes place because the abundant release of Cl-ions from HCl in solution allows the. Rinse three to four times with deionized water before putting the glassware away. First of all solid NaOH absorbs water from the air so accurately weighing a sample during the preparation of a solution is impossible.
 Source: youtube.com
Source: youtube.com
And research shows that when subjects consume the same amounts of creatine HCL and creatine monohydrate the creatine HCL is absorbed by the intestines around 60 better than creatine monohydrate. Answer 1 of 5. This is in fact how decaffeinated tea and coffee were industrially produced for many years. Water breaks the ionic bond by hydrogen bonding as water itself has a more ionic bond and is polar in nature. An organic compound which is soluble in water is typically a low molecular weight polar compound of up to 5-6 carbon atoms or less.

Why and how do you say AgCl is not soluble in water. Peas and beans are very poor sources of fat soluble vitamins and rich sources of water soluble vitamins. An acid-base extraction is a modification of case 2. This will eliminate impurities in the precipitate without solubilizing again the chlorides thats why is added with some HCl. Since most of the extractions are performed using aqueous solutions ie 5 NaOH 5 HCl the miscibility of the solvent with water is a crucial point as well as the compatibility of the reagent with the compounds and the solvent of the solution to be extracted.
 Source: slideshare.net
Source: slideshare.net
An acid-base extraction is a modification of case 2. Many other solvents such as kerosene and petrol are not capable of breaking the ionic bond. Creatine HCL has been shown in the lab to be about 40 times more soluble in fluid than creatine monohydrate. Once the NaOH solution has been standardized and its concentration is well-known it can be used to titrate other acid solutions such as HCl. And research shows that when subjects consume the same amounts of creatine HCL and creatine monohydrate the creatine HCL is absorbed by the.
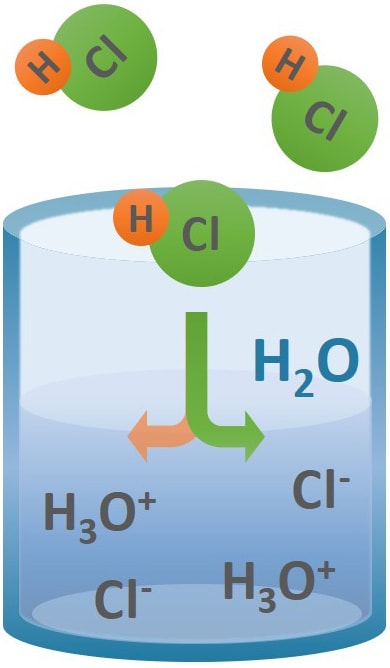 Source: protank.com
Source: protank.com
Rinse three to four times with deionized water before putting the glassware away. EDTA is insoluble in water at low pH because H4Y is predominant in that pH less than 2. Amines with less molecular masses are soluble. Many soluble in water but not in non-polar liquid Classify the following as metal or non-metal and ionic or covalent compounds. However it can be used for people who experience stomach upset with creatine monohydrate.
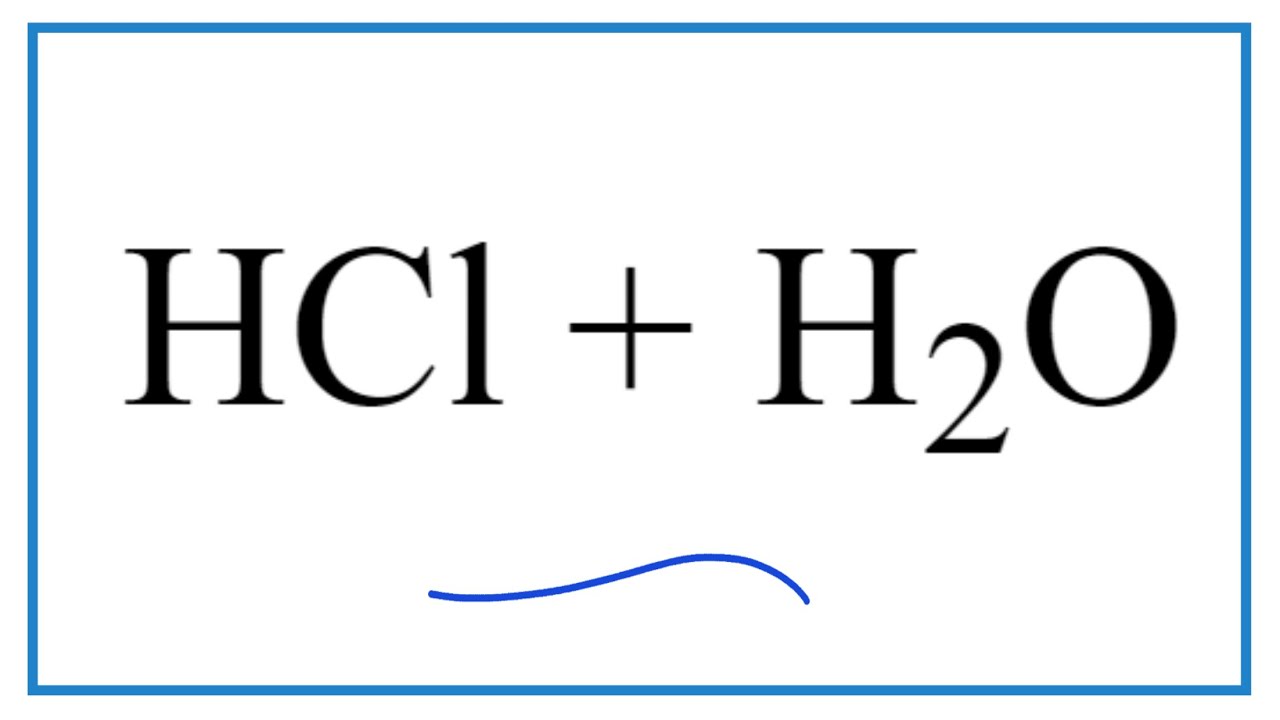 Source: youtube.com
Source: youtube.com
The water soluble vitamins. Amides with less molecular masses small alkyl groups are soluble. With increasing the pH each hydrogen ion in the carboxyl groups of EDTA will start to dissociate. And research shows that when subjects consume the same amounts of creatine HCL and creatine monohydrate the creatine HCL is absorbed by the intestines around 60 better than creatine monohydrate. With better absorption and.

The sodium cations and the chloride anions act as spectator ions because. The equation for this reaction is BaOH2 2 HCl BaCl2 2 H2O. The sodium cations and the chloride anions act as spectator ions because. The term pulse is commonly used. Covalent MgCl 2 H 2 O CCl 4 HF Hydrogen or H is this.
 Source: slideplayer.com
Source: slideplayer.com
This is in fact how decaffeinated tea and coffee were industrially produced for many years. Soluble in water Insoluble in kerosene. Reacts with water and form soluble HCl and carboxylic acid. If you want to get proved AgCl is insoluble in water compare its solubility and NaCls solubility in water. More specifically you will have NaOH_ aq - Na_ aq OH_ aq- HCl_ aq - H_ aq Cl_ aq- Now when these two solutions are mixed the hydroxide anions produced by the strong base and the hydrogen ions produced by the strong acid will neutralize each other to produce water.

Include the ionic reaction and the expression for K sp in your answer. Once the NaOH solution has been standardized and its concentration is well-known it can be used to titrate other acid solutions such as HCl. Many other solvents such as kerosene and petrol are not capable of breaking the ionic bond. How to precipitate chlorides of Group I Cations. Soluble in water Insoluble in kerosene.
 Source: youtube.com
Source: youtube.com
Include the ionic reaction and the expression for K sp in your answer. Why and how do you say AgCl is not soluble in water. We are 2 miles north of the US. In some countries various terms are often substituted for legume. We have the full infrastructure in place including roads highways nearby rail electrical power water and natural gas.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why is hcl soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.