Why is butanol insoluble in water
Home » chemistry » Why is butanol insoluble in waterWhy is butanol insoluble in water
Why Is Butanol Insoluble In Water. A solvent from the Latin solvō loosen untie solve is a substance that dissolves a solute resulting in a solutionA solvent is usually a liquid but can also be a solid a gas or a supercritical fluidWater is a solvent for polar molecules and the most common solvent used by living things. For example the. A 2050102 M solution of NaCl in water is at 200C. 4Saffron macerated with water imparts a yellow colour in the aqueous phase.
 Solubility Of Water In The Organic Phase And Selectivity Of The Il Download Scientific Diagram From researchgate.net
Solubility Of Water In The Organic Phase And Selectivity Of The Il Download Scientific Diagram From researchgate.net
1-Butanol 2 2 1 2-Butanol 2 4-DNP Test for Aldehydes and Ketones 1 acetates 1 Acetone 1 Acetone Test 1 acidimetry and alkalimetry 1 activation energy 1 Alternatives for Pesticides 2 Analysis of an antacid tablet 1 Andrews condition 1 antacid When the reaction involves in a titration does not satisfies the conditions for a direct titration to be performed 1 aryl 1. Cellulose fibers are enmeshed in a. Phenol can make hydrogen bonds due to -OH bond. The woodglue cracks after flexing. Carbon chain on the other hand as nonpolar is repelled. Predict the solubility of these two compounds in 10 aqueous hydrochloric acid.
Carboxylic acids are a group of organic compounds which have weakly acidic properties.
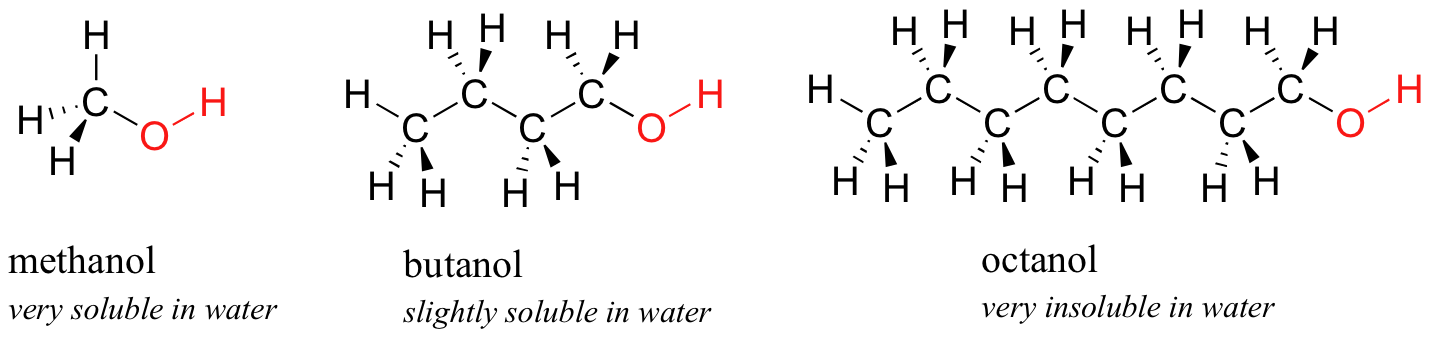
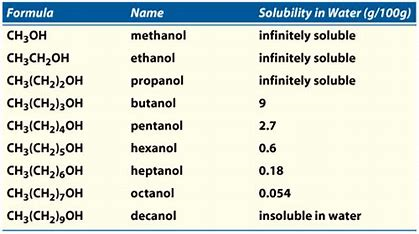
Methanol ethanol dissolve very well in water. A The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride. 6Thin layer chromatography Spot 20 μl of 5 aqueous extract of the sample on a silica gel G plate using a mixture of n-butanol acetic acid and. View Answer A 722 moll aqueous solution of NaI is 60 percent NaI by. After clearing the column with benzene and then chloroform 5 ml of Carr-Price reagent turns the band violet. A 2050102 M solution of NaCl in water is at 200C.
 Source: researchgate.net
Source: researchgate.net
Bases are insoluble substances which neutralize acids to form a salt and water only and are proton acceptors Alkalis turn red litmus indicator paper or solution to blue. 1-Butanol 2 2 1 2-Butanol 2 4-DNP Test for Aldehydes and Ketones 1 acetates 1 Acetone 1 Acetone Test 1 acidimetry and alkalimetry 1 activation energy 1 Alternatives for Pesticides 2 Analysis of an antacid tablet 1 Andrews condition 1 antacid When the reaction involves in a titration does not satisfies the conditions for a direct titration to be performed 1 aryl 1. Cellulose supports the cell wall of plants. Cellulose is a structural protein in plants and algae. A The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
 Source: researchgate.net
Source: researchgate.net
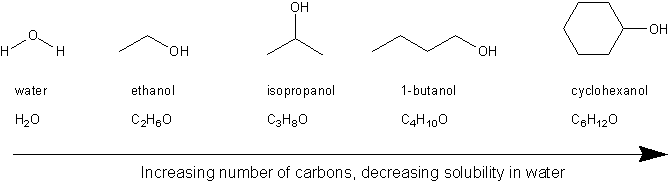
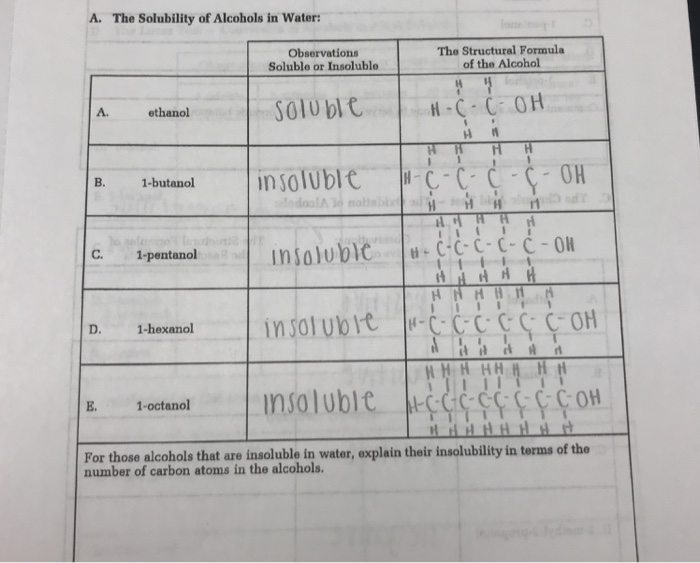
The sample was created by dissolving a sample of NaCl in water and then bringing the volume up to 1000 L. Agitate the tubes to mix the contents. Has more C atoms a longer chain in this series then this interaction is. You will have to look up the structure of this compound and comment on why it is water-soluble. Thus whereas the hydrocarbons are insoluble in water small alcohols with one to three carbon atoms are completely soluble.
 Source: chem.libretexts.org
Source: chem.libretexts.org
They dissolve in water in any amount. Let the aspirator run for a few minutes to start air-drying the crystals. The water should filter quickly - if not check for vacuum leaks. They react with alcohols another organic series of compounds to form organic compounds called esters which are used as solvents and components in perfumes and food flavourings. Label clean dry test tubes as follows.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Methanol ethanol dissolve very well in water. A solvent from the Latin solvō loosen untie solve is a substance that dissolves a solute resulting in a solutionA solvent is usually a liquid but can also be a solid a gas or a supercritical fluidWater is a solvent for polar molecules and the most common solvent used by living things. As the length of the chain increases however the solubility of alcohols in water decreases. Starting with the four-carbon butanol the. Carboxylic acids have high boiling points compared to other substances of comparable.
 Source: mendelset.com
Source: mendelset.com
It was determined that the volume of water needed to do this was 9994 mL. Place 10 drops 05 ml 05 g of the appropriate compound into each test tube. When alkyl group gets longer or larger solubility of alcohols decreases. Its basic application is as a binder in tablet formulations. Ttsz Getty Images.
 Source: chegg.com
Source: chegg.com
The octyl acetate and benzyl acetate produce milky emulsions which should be given five minutes to separate. Experimental Procedure Step I - Measure 10 mL of. A The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride. You will have to look up the structure of this compound and comment on why it is water-soluble. When completely immersed in water however it has a weight of W_textin water 48 N.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A solvent from the Latin solvō loosen untie solve is a substance that dissolves a solute resulting in a solutionA solvent is usually a liquid but can also be a solid a gas or a supercritical fluidWater is a solvent for polar molecules and the most common solvent used by living things. Explain why. The upper layer is the ester which is a water-insoluble volatile liquid. Superabsorbent hydrogels SAHs are biomaterials with high capacity of absorption and retaining of large amount of water saline and physiological solutions at least 1020 times of their molecular weight SAHs consist of a three-dimensional network of hydrophilic homo- or co-monomers which are insoluble in water due to anionic interactions and hydrogen bonding. Base acid salt water CO2 when base is a metal carbonate Base ammonium salt salt ammonia gas water Strong alkalis completely ionize in water producing lots of OH- ions Weak alkalis.
 Source: researchgate.net
Source: researchgate.net
Get all the crystals out of the flask using a spatula or stirring rod. If released as water it has the potential to impact our waterways and impacts. Would you expect butyric acid butanoic acid to be more or less soluble than 1-butanol in water. Cellulose fibers are enmeshed in a. They react with alcohols another organic series of compounds to form organic compounds called esters which are used as solvents and components in perfumes and food flavourings.
 Source: socratic.org
Source: socratic.org
5It gives a pale yellow colour to ether or petroleum ether. For example the. The react with basesalkalis to for salts and release carbon dioxide from carbonates. Rinsing with 1 or 2 mLs of cold water helps get the crystals out of the flask and rinsing helps remove impurities. Propanol dissolve little bit.
 Source: saylordotorg.github.io
Source: saylordotorg.github.io
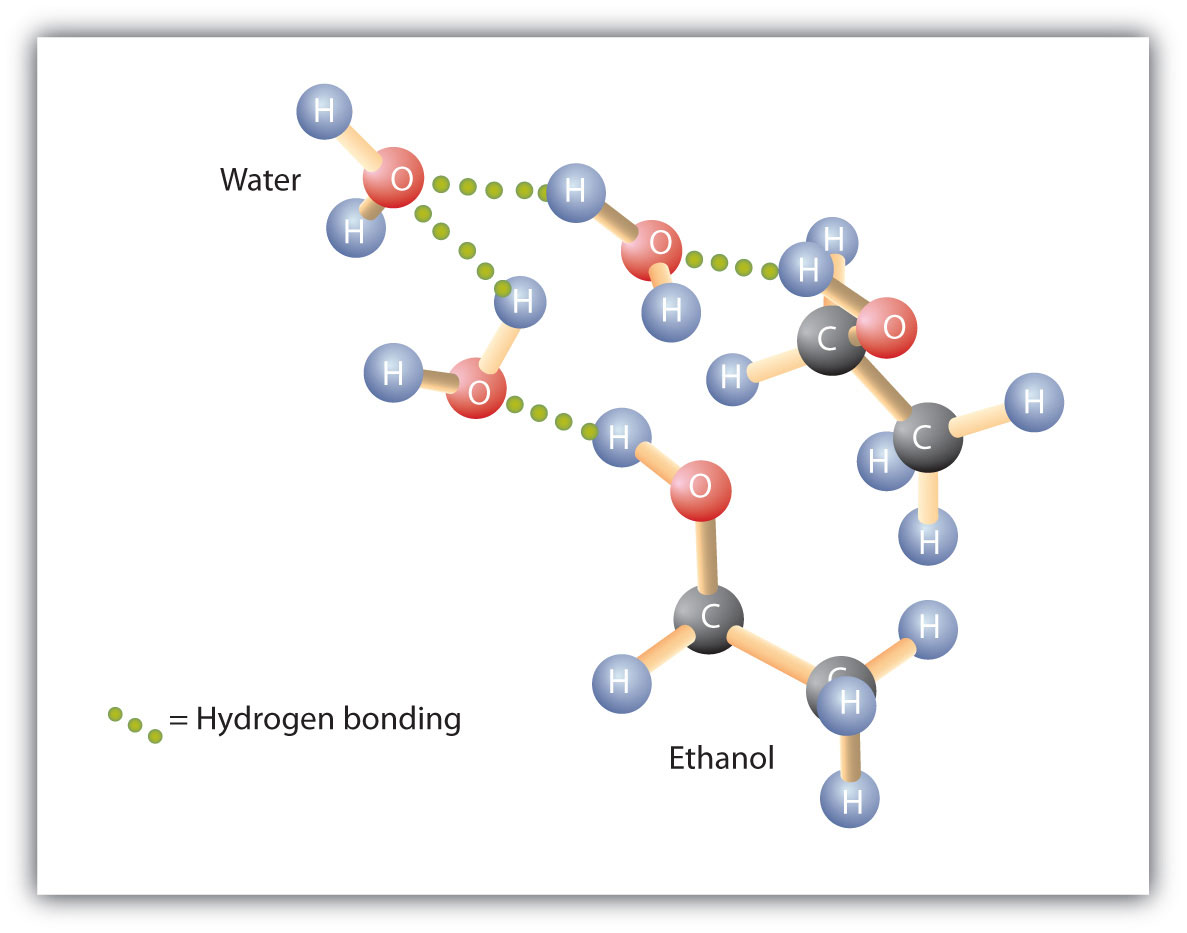
Note that in general liquids that. Allow the glue to dry before moving on to the next stage. Because of the strength of the attraction of the OH group first three alcohols methanol ethanol and propanol are completely miscible. 1-propanol 1-butanol 1-pentanol phenol 5-isopropyl-2-methylphenol. Decide on a classification for each of the vitamins shown below.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why is butanol insoluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.