Why is benzoic acid insoluble in water
Home » chemistry » Why is benzoic acid insoluble in waterWhy is benzoic acid insoluble in water
Why Is Benzoic Acid Insoluble In Water. The first five entries all have oxygen functional groups and the relatively high boiling points of the first two is clearly due to hydrogen bonding. This produces a water-soluble pleasant-smelling white powder that is used for flavorings and perfume. The ether solution of the alcohol may then be separated from the water layer and pure alcohol. Answer 1 of 3.
 Temperature Dependent Solubility Of Benzoic Acid In Aqueous Phase And Aqueous Mixtures Of Aliphatic Alcohols From degruyter.com
Temperature Dependent Solubility Of Benzoic Acid In Aqueous Phase And Aqueous Mixtures Of Aliphatic Alcohols From degruyter.com
Explain why a Büchner or Hirsh funnel is used to isolate the final crystallized product instead of stem funnel. The sample is insoluble in water. 1 Weigh 4 g of the crude benzoic acid mixture using the. Take about 200ml of dye solution and dip in it the woolen cloth to be dyed. Are insoluble in water. To convert moles to grams and grams to milliliters.
Before you come to the laboratory do the Pre-Lab assignments for this laboratory as assigned by your.
The ether solution of the alcohol may then be separated from the water layer and pure alcohol. Academiaedu is a platform for academics to share research papers. Benzoic acids antimicrobial activity is primarily against yeasts and. What is an effective method for purification of volatile solids. Acetic acid however is quite soluble. The name malic acid comes from the apples botanical genus name malus while lactic acid HC 3 H 5 O 3 is found in wine and sour milk products such as yogurt and some cottage cheeses.
 Source: worldofchemicals.com
Source: worldofchemicals.com
This produces a water-soluble pleasant-smelling white powder that is used for flavorings and perfume. The first five entries all have oxygen functional groups and the relatively high boiling points of the first two is clearly due to hydrogen bonding. Are volatile in steam. It occurs naturally in prunes cinnamon and cloves. This includes the melting point and solubilities in water and ether of benzoic acid and C O OH Benzoic Acid Naphthalene.

The first five entries all have oxygen functional groups and the relatively high boiling points of the first two is clearly due to hydrogen bonding. The depression in freezing point of water observed for the same amount of acetic acid trichloroacetic acid and trifluoroacetic acid increases in the order given above. When the mouse pointer passes over the. The neutralising reaction can produce two salts - either the monosodium variety generally known simply as sodium salicylate where the proton from the carboxylic acid group is donated or the disodium one where both available protons are donated. Finally the benzoic acid will be precipitated by adding strong acid to the carboxylate salt solution.
 Source: researchgate.net
Source: researchgate.net
The most familiar example is a mixture of ethanol and water in the ratio of 9587. Calculate the volume of boiling water needed to dissolve 10 g of benzoic acid. HCl H 2 O H 3 O Cl When the two solutions are mixed the H. NaOH Na OH and similarly in water the acid hydrogen chloride forms hydronium and chloride ions. You will use a chemically active extraction to convert the water insoluble benzoic acid into its water soluble salt by treating the carboxylic acid with base.
 Source: ochemonline.wordpress.com
Source: ochemonline.wordpress.com
Are volatile in steam. Are volatile in steam. To make 0011 moles of ester therefore 0011 moles of alcohol and 0012 moles of carboxylic acid are needed. The most familiar example is a mixture of ethanol and water in the ratio of 9587. CHEM 2423 Recrystallization of Benzoic Acid Dr.
 Source: degruyter.com
Source: degruyter.com
Are volatile in steam. Explain why o-chlorobenzoic acid is insoluble in water but sodium o-chlorobenzoate is soluble in water. Techniques and Transformations 2 naphthalene as well as the boiling point solubility in water and density of diethyl ether or tert-butyl methyl ether. Are volatile in steam. Are insoluble in water.
 Source: youtube.com
Source: youtube.com
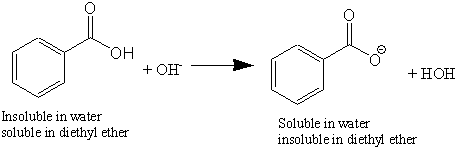
Calculate the amount of benzoic acid C 5 H 5 COOH required for preparing 250 mL of 0 15 M solution in methanol. The depression in freezing point of water observed for the same amount of acetic acid trichloroacetic acid and trifluoroacetic acid increases in the order given above. This is easy to explain using the small alcohol vs large alcohol argument. Assuming the solution is chilled at 0. For example if a solution of benzoic acid pK a 42 in benzyl alcohol pK a 15 is dissolved in ether and shaken with an excess of 01 N sodium hydroxide pH 13 the acid is completely converted to its water soluble ether insoluble sodium salt while the alcohol is unaffected.
 Source: researchgate.net
Source: researchgate.net
Finally the benzoic acid will be precipitated by adding strong acid to the carboxylate salt solution. It boils at 7813 o C. Although the balanced reaction shows that 1 mole of alcohol reacts with 1 mole of carboxylic acid to yield 1 mole of ester plus 1 mole of water a slight excess of carboxylic acid is used an additional 0001 mole. Benzoic acid was also recrystallized with a 79 recovery using water as the solvent. The free acid form is poorly soluble in water and the sodium salt sodium benzoate is often used because of its greater solubility Fig.

Benzoic acids antimicrobial activity is primarily against yeasts and. Warming results in a clear solution of the dye. Its also rather nontoxic and no flammable solvents are involved. For water we suggest 15 µgL the EPA Action Level in water and for air the National Ambient Air Quality Standard of 015 µgm 3. Determine the major organic product for the following reaction scheme chegg.
 Source: oneclass.com
Source: oneclass.com
Before you come to the laboratory do the Pre-Lab assignments for this laboratory as assigned by your. There are several reasons why nonaqueous acid-base titrations may be used instead of aqueous titrations. This is easy to explain using the small alcohol vs large alcohol argument. For example if a solution of benzoic acid pK a 42 in benzyl alcohol pK a 15 is dissolved in ether and shaken with an excess of 01 N sodium hydroxide pH 13 the acid is completely converted to its water soluble ether insoluble sodium salt while the alcohol is unaffected. Take about 01g of malachite green dye and add to it 400ml of water.
 Source: home.miracosta.edu
Source: home.miracosta.edu
Benzoic Acid Use of two different bases with two different strengths allows for selective reaction of the stronger acid versus the weaker acid. Determine the major organic product for the following reaction scheme chegg. The depression in freezing point of water observed for the same amount of acetic acid trichloroacetic acid and trifluoroacetic acid increases in the order given above. If you want to practice benzoic acid. The first five entries all have oxygen functional groups and the relatively high boiling points of the first two is clearly due to hydrogen bonding.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title why is benzoic acid insoluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.