Which cu 2 complexes soluble or insoluble
Home » chemistry » Which cu 2 complexes soluble or insolubleWhich cu 2 complexes soluble or insoluble
Which Cu 2 Complexes Soluble Or Insoluble. Plenum Press 1978 p. 2 CuNO 3 2 2 CuO 4 NO 2 O 2 3 NO 2 H 2 O 2HNO 3 NO. Iron chelators treat iron overload a condition often caused by transfusion therapies used to treat thalassemias and other anemias. These films may be porous and not perfectly adherent in this case resulting in.
 Copper Cu Transition Metal Chemistry Copper I Cu Copper Ii Cu2 Ion Complex Ions Ligand Substitution Compounds Redox Chemical Reactions Principal Oxidation States 1 2 Gce As A2 Ib A Level Inorganic Chemistry Revision From docbrown.info
Copper Cu Transition Metal Chemistry Copper I Cu Copper Ii Cu2 Ion Complex Ions Ligand Substitution Compounds Redox Chemical Reactions Principal Oxidation States 1 2 Gce As A2 Ib A Level Inorganic Chemistry Revision From docbrown.info
The Chemical and Products Database a resource for. Plenum Press 1978 p. CuBr SCH 3 2 CuBrSCH 3 2 In this. Cu s Cu 2 aq 2 Cl aq. For Cu valence state the high-resolution Cu 2p XPS spectra of both Cu-CDs and Na 2 CuEDTA showed the peaks at 9325 eV 2p 32 and 9525 eV 2p 12 respectively which are associated with. Here chelating ligands are often used to form insoluble complexes eg.
These films may be porous and not perfectly adherent in this case resulting in.
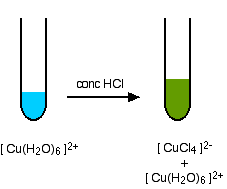
Similarly the solubility of CuCl 2 is affected to a certain extent by the presence of other chlorides in the solution. CopperI chloride is made by boiling a solution of HCl aq and CuCl 2aq in the presence of excess Cu. B Gravimetric Analysis. C Complexometric Titrations and Masking Agents. Here chelating ligands are often used to form insoluble complexes eg. In the solid stability depends on the neighboring anion and the resulting lattice energy of the ionic solid.
 Source: docbrown.info
Source: docbrown.info
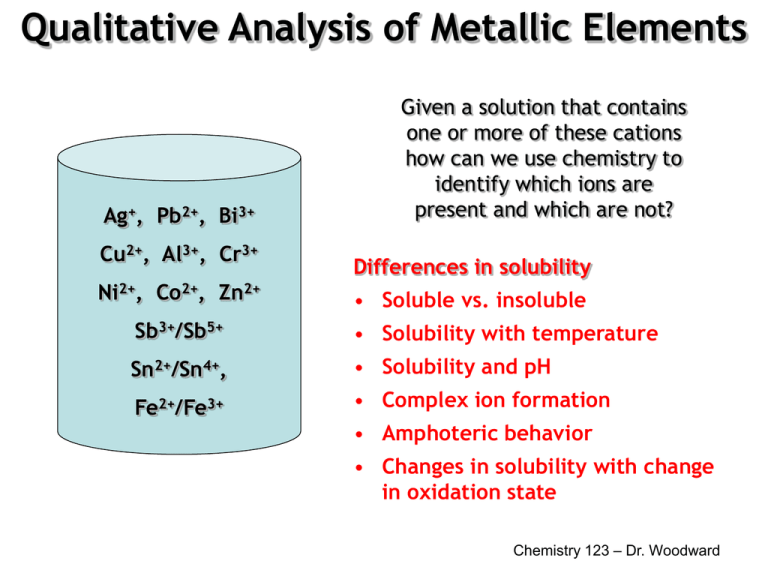
Cu s Cu 2 aq 2 Cl aq. Fe 2 forms weak complexes in the chloride medium and consequently is a donor of Cl ions. For the separation and detection of the cations you will use the ability of these ions to form precipitates to form complex ions or to show amphoteric behavior act as either an acid or a base. Additionally approximately 22 of total body iron is found in the so-called labile pool a poorly defined and reactive pool of iron that forms reactive oxygen species via the Fenton Reaction which forms complexes with a drug class known as chelators. Possible ions are Ag Cu 2 Fe 3 Cr 3 Zn 2 and Ba 2.
 Source: docbrown.info
Source: docbrown.info
The insoluble complex is excreted without being absorbed from the intestinal walls. These films may be porous and not perfectly adherent in this case resulting in. Are insoluble in water. Here chelating ligands are often used to form insoluble complexes eg. Hazardous Substances Data Bank HSDB 8 Use and Manufacturing.
 Source: chemguide.co.uk
Source: chemguide.co.uk
CuBr SCH 3 2 CuBrSCH 3 2 In this. Here chelating ligands are often used to form insoluble complexes eg. Copper complexes of some amino acids are easily absorbed. On the other hand the Zn 2 ion forms strong complexes and therefore acts as an acceptor of Cl. B Gravimetric Analysis.
 Source: docbrown.info
Source: docbrown.info
Zn 2 Pb 2 Ca 2 Co 2 Ni 2 Cu 2 etc. For example with dimethyl sulfide the colorless complex is formed. For Cu valence state the high-resolution Cu 2p XPS spectra of both Cu-CDs and Na 2 CuEDTA showed the peaks at 9325 eV 2p 32 and 9525 eV 2p 12 respectively which are associated with. Synthesis takes advantage of the stability of solid CuCl which makes redox between Cu o and Cu 2 spontaneous. CopperI chloride is made by boiling a solution of HCl aq and CuCl 2aq in the presence of excess Cu.
 Source: studylib.net
Source: studylib.net
Synthesis takes advantage of the stability of solid CuCl which makes redox between Cu o and Cu 2 spontaneous. Metal Toxicity in Mammals 2. We would like to show you a description here but the site wont allow us. Cu s Cu 2 aq 2 Cl aq. Fe 2 forms weak complexes in the chloride medium and consequently is a donor of Cl ions.
 Source: chemguide.co.uk
Source: chemguide.co.uk
CuBr SCH 3 2 CuBrSCH 3 2 In this. Plenum Press 1978 p. B Gravimetric Analysis. EPA CPDat Chemical and Product Categories. The group V ligand is oxidized to the oxide.
 Source: youtube.com
Source: youtube.com
Insoluble prussian blue decreases the half life of cesium by 33 and from 38 to 22 days for thallium. Synthesis takes advantage of the stability of solid CuCl which makes redox between Cu o and Cu 2 spontaneous. Upon treatment with Lewis bases CuBr converts to molecular adducts. Possible ions are Ag Cu 2 Fe 3 Cr 3 Zn 2 and Ba 2. However its solubility in water is considerably higher than that of CuCl.
 Source: slidetodoc.com
Source: slidetodoc.com
EPA CPDat Chemical and Product Categories. Treatment of copperII nitrate solutions with triphenylphosphine triphenylarsine and triphenylstibine gives the corresponding copperI complexes CuEC 6 H 5 3 3NO 3 E P As Sb. For example with dimethyl sulfide the colorless complex is formed. These films may be porous and not perfectly adherent in this case resulting in. Insoluble prussian blue decreases the half life of cesium by 33 and from 38 to 22 days for thallium.
 Source: chemguide.co.uk
Source: chemguide.co.uk
Cu s Cu 2 aq 2 Cl aq. Zn 2 Pb 2 Ca 2 Co 2 Ni 2 Cu 2 etc. NiDMG 2 and Aloxine 3 see laboratory manual. In the first week you do preliminary tests with each cation to discover each ions characteristic behavior. Fe 2 forms weak complexes in the chloride medium and consequently is a donor of Cl ions.
 Source: nature.com
Source: nature.com
An example of this is the use of EDTA in the volumetric determination of a wide variety of metal ions in solution eg. Metal Toxicity in Mammals 2. EPA CPDat Chemical and Product Categories. Fe 2 forms weak complexes in the chloride medium and consequently is a donor of Cl ions. The rate of cesium and thallium elimination is proportional to the dose and duration of prussian blue.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title which cu 2 complexes soluble or insoluble by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.