Two other insoluble copper salts
Home » chemistry » Two other insoluble copper saltsTwo other insoluble copper salts
Two Other Insoluble Copper Salts. Compared to other trace elements relatively little Cu is stored in the body. CuO H2SO4 CuSO4 H2O Step 2 Filter off the excess copperII oxide using a filter paper and funnel. Copper sulfate is blue because of the copperII. The presence of oxygen in the acidic solution causes.
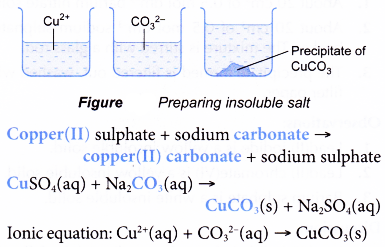 Describe The Preparation Of Soluble And Insoluble Salts A Plus Topper From aplustopper.com
Describe The Preparation Of Soluble And Insoluble Salts A Plus Topper From aplustopper.com
When solutions of two soluble salts are mixed a solid may form. 5A white coloured powder is used by doctors for supporting. Sharpless in 2001 to describe reactions that are high yielding wide in scope create only byproducts that can be removed without chromatography are stereospecific simple to perform and can be conducted in easily removable or benign solvents. CuO H2SO4 CuSO4 H2O Step 2 Filter off the excess copperII oxide using a filter paper and funnel. The recrystallized salt is a higher quality and higher purity because insoluble mineral matter and other insoluble impurities were left in the ground. Due to interaction of your buffer components with other solution components.
A Those substances whose smell odour changes in acidic or basic solution are called olfactory indicators eg.
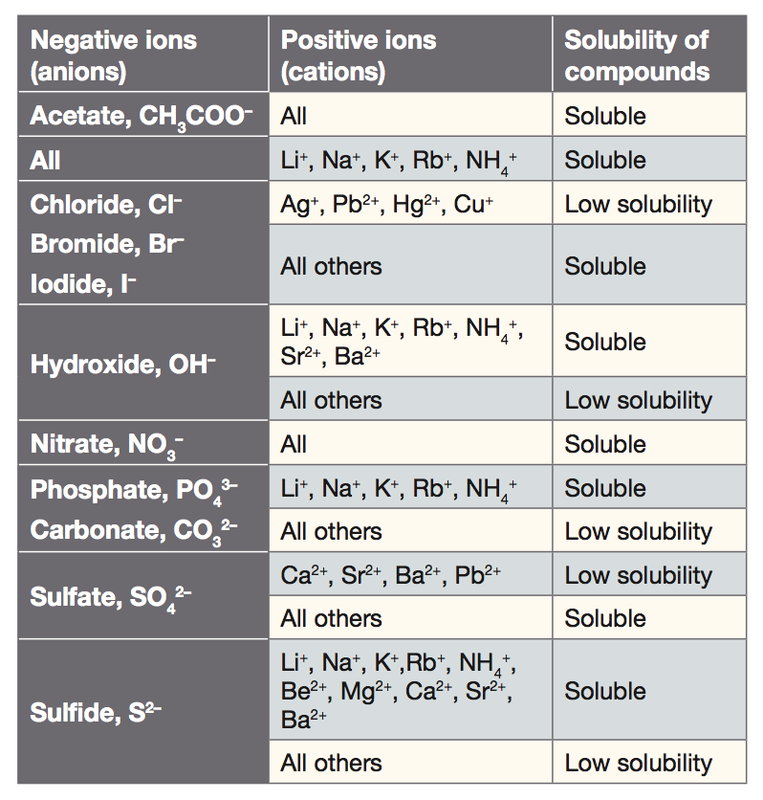
Some organic dyes are salts but they are virtually insoluble in water. Many salts contain water molecule and are known as Hydrated Salts. Hydroxide is a diatomic anion with chemical formula OH It consists of an oxygen and hydrogen atom held together by a single covalent bond and carries a negative electric chargeIt is an important but usually minor constituent of waterIt functions as a base a ligand a nucleophile and a catalystThe hydroxide ion forms salts some of which dissociate in aqueous solution liberating. Compared to other trace elements relatively little Cu is stored in the body. The insoluble salt that falls out of solution is known as the precipitate hence the reactions name. Soluble salts which are not ammonium sodium and potassium salts can be prepared by reacting dilute acids with insoluble metals bases or carbonates.

Copper is an essential nutrient required for a number of metabolic reactions. Standard vehicle traffic and other than standard vehicle traffic eg. Among numerous metal salts used with these dyes chromium and copper are most common. CuO H2SO4 CuSO4 H2O Step 2 Filter off the excess copperII oxide using a filter paper and funnel. The insoluble reactant chosen depends upon the particular salt required.
 Source: savemyexams.co.uk
Source: savemyexams.co.uk
When it is mixed with calcium hydroxide it is known as Bordeaux mixture. Does BaSO4 form a precipitate. In the formation of bone this is a normal condition. 38 Preparation of copperII sulphate acid on insoluble base Chapter 10 Acids Bases and Salts Step 1 Place about 50 cm³ of dilute sulphuric acid in a beaker and gently warm the acid. Soluble salts which are not ammonium sodium and potassium salts can be prepared by reacting dilute acids with insoluble metals bases or carbonates.
 Source: savemyexams.co.uk
Source: savemyexams.co.uk
The insoluble reactant chosen depends upon the particular salt required. Lead chloride is a crystalline salt whose formation is encouraged by micro holes in the wall larger. The insoluble reactant chosen depends upon the particular salt required. Soluble salts which are not ammonium sodium and potassium salts can be prepared by reacting dilute acids with insoluble metals bases or carbonates. When copper sulphate is heated it loses.
 Source: savemyexams.co.uk
Source: savemyexams.co.uk
B Choose strong acids from the following. Acid metal salt hydrogen. Processed food samples are normally dried ground and then dispersed in hot 80 ethanol solutions. On the other hand oxidizing acids such as nitric acid and chromic acid increase the corrosion rate of copper. Often the metal ion also unites with the fibre improving the resistance of the dye to washing.
 Source: aplustopper.com
Source: aplustopper.com
The remelting step reduces the potassium content to less than 100 parts per million. Copper is an essential nutrient required for a number of metabolic reactions. When it is mixed with calcium hydroxide it is known as Bordeaux mixture. B Choose strong acids from the following. In the formation of bone this is a normal condition.
 Source: chemistryrules.me.uk
Source: chemistryrules.me.uk
Copper absorption is proposed to occur by two mechanisms one energy- dependent and the other enzymatic. Factors that can interfere with copper absorption include competition for binding sites with zinc interactions with molybdenum and sulfates chelation with phytates and inhibition by ascorbic acid. The starch granules are water-insoluble and have a relatively high density 1500 kgm 3 so that they will tend to move to the bottom of a container during centrifugation where they can be separated from the other water-soluble and less dense materials. B H 2 SO 4 and HNO 3 are strong acids. Some organic dyes are salts but they are virtually insoluble in water.
 Source: docbrown.info
Source: docbrown.info
CopperII oxide is added a little at a time to the acid until no more can dissolve. Lithium metal which can be drawn into wire and rolled into sheets is softer than lead but harder than the other alkali metals and has the body-centred cubic. Are chemical elements that are required in very small trace amounts in the diet to maintain health. B Choose strong acids from the following. Standard vehicle traffic and other than standard vehicle traffic eg.
 Source: anglesandacid.com
Source: anglesandacid.com
For example copper selenium and iodine are considered trace elements. Lead chloride is a crystalline salt whose formation is encouraged by micro holes in the wall larger. Lithium metal which can be drawn into wire and rolled into sheets is softer than lead but harder than the other alkali metals and has the body-centred cubic. For example copper does not react with dilute acids so copper salts are made using copper oxide or copper carbonate not. Some organic dyes are salts but they are virtually insoluble in water.
 Source: docbrown.info
Source: docbrown.info
Emphysema a chronic obstructive pulmonary lung disease characterized by damage to the small air sacs. Therefore the corrosion rate of copper in HCl particularly in moderate concentration is higher than H 2 SO 4 which is due to the formation of soluble compounds such as CuCl 2. Click Chemistry Azide-Alkyne Cycloaddition Click Chemistry is a term that was introduced by K. 38 Preparation of copperII sulphate acid on insoluble base Chapter 10 Acids Bases and Salts Step 1 Place about 50 cm³ of dilute sulphuric acid in a beaker and gently warm the acid. The insoluble salt that falls out of solution is known as the precipitate hence the reactions name.
 Source: tes.com
Source: tes.com
Blue colour of copper sulphate is due to presence of 5 molecules of water. CuO H2SO4 CuSO4 H2O Step 2 Filter off the excess copperII oxide using a filter paper and funnel. Copper absorption is proposed to occur by two mechanisms one energy- dependent and the other enzymatic. Lead chloride is a crystalline salt whose formation is encouraged by micro holes in the wall larger. On the other hand oxidizing acids such as nitric acid and chromic acid increase the corrosion rate of copper.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title two other insoluble copper salts by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.