Nickel chloride soluble in water
Home » chemistry » Nickel chloride soluble in waterNickel chloride soluble in water
Nickel Chloride Soluble In Water. What are the precipitates. Mixtures of this compound in air with methanol. When ammonium sulfate and NaCl solutions react NH4Cl is produced. In this section we learn that type of variations too.
 Is Nicl2 Soluble Or Insoluble In Water Youtube From youtube.com
Is Nicl2 Soluble Or Insoluble In Water Youtube From youtube.com
In this section we learn that type of variations too. Acetate chloride and sulfate whereas cadmium oxide carbonate and sulfide are almost insoluble 1. Sources Cadmium is a relatively rare element 02 mgkg in the earth crust and is not found in the pure state in the nature. This compound is highly water-soluble and mildly acidic. Al 3 aq 6H 2 Ol. Confirmatory tests of Lead II ion Pb 2 a Potassium iodide test.
However when we add an excess of solid AgCl to water it dissolves to a small extent and produces a mixture consisting of a very dilute solution of Ag and Cl ions in equilibrium with undissolved silver chloride.
Nickel nitrate is a green crystalline solid. Both water-insoluble nickel sulfide NiS and water-soluble nickel sulphate NiSO 4 and nickel chloride NiCl 2 are human carcinogens although insoluble nickel compounds are more potent carcinogens than the soluble ones. Common salts of nickel such as chloride nitrate and sulfate dissolve in water to give green. However there is an increasing interest in water-soluble synthetic polymers known to be a source of marine pollution particularly concerning the threats they pose to soil and water surroundings. Relatively large amounts of nickel comparable to the estimated average ingestion. In the presence of water scavengers hydrated nickelII chloride reacts with dimethoxyethane dme to form the molecular complex NiCl 2 dme 2.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sources Cadmium is a relatively rare element 02 mgkg in the earth crust and is not found in the pure state in the nature. The addition of HCl to the solution will precipitate Pb 2 as lead chloride which is soluble in hot water. Mixtures of this compound in air with methanol. Nickel nitrate is a green crystalline solid. Dietary intake is estimated at 70 to 100 µgday with less than 10 absorbed.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Aluminum chloride hydrolyses in water and forms a mist when it comes in contact with air because hydrochloric acid drops form when it reacts with water vapor. Developing alternative methods to eliminate. Relatively large amounts of nickel comparable to the estimated average ingestion. Silver chloride is whats known as a sparingly soluble ionic solid. It is therefore crucial to avoid the addition of too large an excess of the reagent because it may crystallize out with the chelate.
 Source: sciencedirect.com
Source: sciencedirect.com
Al 3 aq 6H 2 Ol. It is noncombustible but it will accelerate the burning of combustible materials. Chapter 3 Chemical Reactions 41 Chemical Equilibrium 7. Green nickel oxide is prepared by firing a mixture of water and pure nickel powder in air at 1000 C or by firing a mixture of high purity nickel powder nickel oxide and water in air. As the polymers are water-soluble they cannot be recovered with the help of normal filtration methods.
 Source: qrdchem.com
Source: qrdchem.com
Some stainless steels copper and nickel. In this section we learn that type of variations too. Titanium compounds generally are not very water soluble. Aluminum ions in other compounds also hydrolyze and this continues until the cationic charge has run out ending the reaction by hydroxide formation. Developing alternative methods to eliminate.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Because precipitate is in the solid phase and deposited at bottom of the solution. Relatively large amounts of nickel comparable to the estimated average ingestion. Single whiskers of green nickel oxide have been made by the closed-tube transport method from oxide powder formed by the decomposition of. It is noncombustible but it will accelerate the burning of combustible materials. Cadmium has only.
 Source: youtube.com
Source: youtube.com
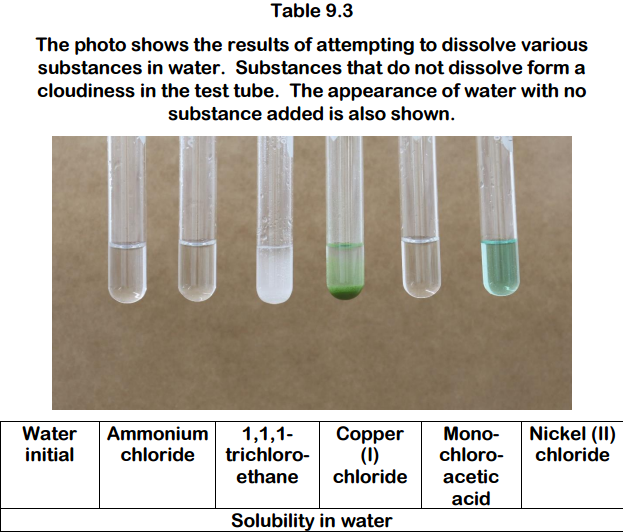
Prolonged exposure to fire or heat may result in an explosion. Recall from the solubility rules in an earlier chapter that halides of Ag are not normally soluble. Common salts of nickel such as chloride nitrate and sulfate dissolve in water to give green. Acutely high intake of soluble Ba-salts nitrates sulfides chlorides can be toxic. It is soluble in water.
 Source: bengislife.com
Source: bengislife.com
It is incompatible with alkali metals. Chronic exposure to Barium may be manifested by muscular and myocardial stimulation tingling in the extremities and loss of tendon reflexes. Chapter 3 Chemical Reactions 41 Chemical Equilibrium 7. In water purification alum. It is soluble in water.
 Source: researchgate.net
Source: researchgate.net
In water purification alum. Some titanium compounds undergo hydrolysis reactions in water for example titanium chloride. HCl solution in hot water reacts with potassium iodide solution to. It is incompatible with amines zinc and alloys of aluminum magnesium and zinc. Green nickel oxide is prepared by firing a mixture of water and pure nickel powder in air at 1000 C or by firing a mixture of high purity nickel powder nickel oxide and water in air.
 Source: bartleby.com
Source: bartleby.com
The greater electrical conductivity of the HCl solution at equilibrium indicates a greater concentration of ions H 3 O and Cl- indicating that the HCl solution is more product-favored at equilibrium than the HCO 2 H solution. When ammonium sulfate and NaCl solutions react NH4Cl is produced. Due to its high density Barium is utilized to absorb. In cosmetics and deodorants aluminum chloride may be present as an astringent. Single whiskers of green nickel oxide have been made by the closed-tube transport method from oxide powder formed by the decomposition of.
 Source: youtube.com
Source: youtube.com
Titanium only reacts with water after its protective titanium oxide surface layer is destroyed. Several inorganic cadmium compounds are quite soluble in water eg. Aluminum chloride and sodium hydroxide. Toxic oxides of. The total nickel ion concentration is a.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title nickel chloride soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.