Magnesium phosphate soluble or insoluble
Home » chemistry » Magnesium phosphate soluble or insolubleMagnesium phosphate soluble or insoluble
Magnesium Phosphate Soluble Or Insoluble. Treat the underlying cause. Some magnesium may react with silica to form magnesium silicate. Phosphate Determination as P 2 O 5. Low pill burden high efficacy works in wide range of pH no negative changes on bone histology.
 Is Mg3 Po4 2 Magnesium Phosphate Soluble Or Insoluble In Water From bengislife.com
Is Mg3 Po4 2 Magnesium Phosphate Soluble Or Insoluble In Water From bengislife.com
These divalent cations cause. 500 mg 750 mg 1000 mg chewable tablets. K sp 1071 x. On line 3 and 4Any solid liquid or gas can copied as in onto the lower lines. Soluble in salt solution Magnetic susceptibility χ 16710 6 cm 3 mol 4 H 2 O Hazards R-phrases outdated See TfM R25 See TfM R36 See TfM R37 See TfM R38. NA Except where otherwise noted data are given for materials in their standard state.
The brine is moved one more.
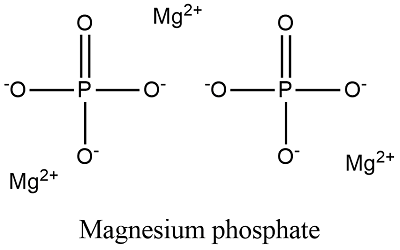
Magnesium is classified as an alkaline earth metal and has 2 hydration shells. CRYSTALS University of Cincinnati MLS Program 7 Amorphous Phosphates Granular colorless Found in alkaline urine Forms white precipitate upon refrigeration Does NOT redissolve upon warming but dissolves in acetic acid. Aluminum salts are often added to water to start precipitation reactions for phosphate removal. A second problem is caused by the presence of calcium and magnesium salts in the water supply hard water. D MgNH 4PO 4 magnesium ammonium phosphate an essentially insoluble substance used in tests for magnesium e Ca 5 PO 4 3 OH the mineral apatite a source of phosphate for fertilizers Hint. Other aluminum compounds are applied in paper production.
 Source: researchgate.net
Source: researchgate.net
Fat soluble vitamins are stored in the body tissues and if your dogs gets too much of a fat soluble vitamin a vitamin excess can accumulate over time. Conversely studies on the influence of absolute fat mass on Mg 2 absorption have not produced consistent results 68-70 reviewed in. Magnesium Carbonate is nearly insoluble however in the presence of stomach acid HCl it is converted to magnesium chloride. Give phosphate binders eg aluminium hydroxide calcium carbonate. NFPA 704 fire diamond 1.

Enhance how intestines absorb calcium zinc phosphate iron and magnesium. Aluminum salts are often added to water to start precipitation reactions for phosphate removal. Solutions of alkali metal soaps are slightly alkaline pH 8 to 9 due to hydrolysis. Magnesium sulphate is reacted upon by caustic soda to form a precipitate of magnesium hydroxide. Forms insoluble phosphate complexes in the gut.
 Source: youtube.com
Source: youtube.com
Other aluminum compounds are applied in paper production. Phosphate Determination as P 2 O 5. There is a 21 ratio between the concentation of the phosphate ion and the molar solubility of the magnesium phosphate. Insoluble impurities such as sand and clay and slightly soluble impurities such as calcium carbonate settle to the bottom as evaporation begins. Calcifications in the skin.
 Source: studylib.net
Source: studylib.net
Allows blood to clot. 1 soluble or insoluble and 2 fermentable or non-fermentable. Enhance how intestines absorb calcium zinc phosphate iron and magnesium. Magnesium is the eighth most common element in the crust of the Earth 1 2 and is mainly tied up within mineral deposits for example as magnesite magnesium carbonate MgCO 3 and dolomiteDolomite CaMgCO 3 2 is as the name suggests abundant in the Dolomite mountain range of the Alps The most plentiful source of biologically available magnesium however is the. NA Except where otherwise noted data are given for materials in their standard state.
 Source: bengislife.com
Source: bengislife.com
Aluminum salts are often added to water to start precipitation reactions for phosphate removal. Insoluble Insoluble Soluble at 60C with acid Soluble Soluble Soluble Slowly change to uric acid Soluble effervesces. Magnesium is classified as an alkaline earth metal and has 2 hydration shells. Magnesium phosphate Mg3PO42 or Mg3O8P2 CID 24439 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Soluble in salt solution Magnetic susceptibility χ 16710 6 cm 3 mol 4 H 2 O Hazards R-phrases outdated See TfM R25 See TfM R36 See TfM R37 See TfM R38.
 Source: chegg.com
Source: chegg.com
Discontinue phosphate intake dietary or medication. Magnesium Carbonate is nearly insoluble however in the presence of stomach acid HCl it is converted to magnesium chloride. Many studies examined the effect of low or indigestible. These divalent cations cause. Some magnesium may react with silica to form magnesium silicate.
 Source: youtube.com
Source: youtube.com
The fiber in Citrucel with Methylcellulose Fiber is 100 soluble. The remaining brine is moved to yet another pond where the salt settles out as evaporation proceeds. Alloys such as duraluminum. CRYSTALS University of Cincinnati MLS Program 7 Amorphous Phosphates Granular colorless Found in alkaline urine Forms white precipitate upon refrigeration Does NOT redissolve upon warming but dissolves in acetic acid. Conversely studies on the influence of absolute fat mass on Mg 2 absorption have not produced consistent results 68-70 reviewed in.
 Source: en.wikipedia.org
Source: en.wikipedia.org
High PO 4 3-levels cause the formation of an insoluble compound with calcium which can lead to. Water Determination Karl Fischer Titrimetric Method Organic Components. This was attributed to the. Insoluble Solubility product K sp 104 10 24. The brine is moved one more.
 Source: clutchprep.com
Source: clutchprep.com
Give phosphate binders eg aluminium hydroxide calcium carbonate. 1 soluble or insoluble and 2 fermentable or non-fermentable. Some magnesium may react with silica to form magnesium silicate. D MgNH 4PO 4 magnesium ammonium phosphate an essentially insoluble substance used in tests for magnesium e Ca 5 PO 4 3 OH the mineral apatite a source of phosphate for fertilizers Hint. Give phosphate binders eg aluminium hydroxide calcium carbonate.
 Source: softschools.com
Source: softschools.com
Low pill burden high efficacy works in wide range of pH no negative changes on bone histology. Magnesium sulphate is reacted upon by caustic soda to form a precipitate of magnesium hydroxide. Just copy those ions from. Low pill burden high efficacy works in wide range of pH no negative changes on bone histology. The brine is pumped or moved by gravity flow to another pond where calcium sulfate settles out as evaporation continues.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title magnesium phosphate soluble or insoluble by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.