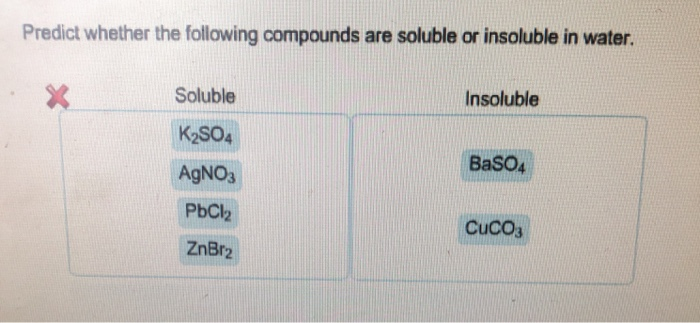
K2so4 soluble or insoluble in water
Home » chemistry » K2so4 soluble or insoluble in waterK2so4 soluble or insoluble in water
K2so4 Soluble Or Insoluble In Water. 15 CopperII hydroxide is a base but not an alkali. It is soluble in water and in the dissolution process it ionizes. This is because it is insoluble in water. Compound Water Soluble Cation Anion a Na2CO3 yes 2.
 Is K2so4 Potassium Sulfate Soluble Or Insoluble In Water From bengislife.com
Is K2so4 Potassium Sulfate Soluble Or Insoluble In Water From bengislife.com
Therefore CuCl2 is soluble. Which one of the following compounds is insoluble in water. View Answer Based on the solubility rules MgNO_3_2 is a Soluble b Insoluble. The chloride ion Cl- is normally soluble and Cu2 is not one of the exceptions to the rule. Potassium carbonate K2CO3 is a white salt soluble in water insoluble in ethanol which forms a strongly alkaline solutionIt can be made as the product of potassium hydroxides absorbent reaction with carbon dioxideIt presents a large capacity to absorb moisture. Find the compound is soluble in hot water and soluble in the cold water of acetanilide.
Compound Water Soluble Cation Anion a Na2CO3 yes 2.
Net ionic equation for kno3 and h2o. Water can dissolve many ionic substances. Search thousands of other internships scholarships and other student programs in 120 countries. Plants readily absorb the K dissolved in the soil water. The salt is soluble in water but insoluble in solutions of potassium hydroxide sp. 1 The mixing of which pair of reactants will result in a precipitation reaction.
 Source: bengislife.com
Source: bengislife.com
1 The mixing of which pair of reactants will result in a precipitation reaction. 1 The mixing of which pair of reactants will result in a precipitation reaction. SECONDARY STAGE CHEMISTRY BOOK ONE FOR CLASS IX. Water as a solvent often plays an active role in the chemical reactions of solutes. This insoluble compound is called a precipitate.
 Source: study.com
Source: study.com
1 The mixing of which pair of reactants will result in a precipitation reaction. In general salts of the alkali metals are soluble. The reaction is. Search thousands of other internships scholarships and other student programs in 120 countries. 1 The mixing of which pair of reactants will result in a precipitation reaction.
 Source: youtube.com
Source: youtube.com
Free essays homework help flashcards research papers book reports term papers history science politics. Water is a molecule. 2 NaC2H302 SrSO BaS AIPOA Select one. Solucionario quimica de raymond chang 12 edicion. When potassium sulfate is heated in water and subject to swirling in a beaker the crystals form a multi-arm spiral structure when allowed to settle.
 Source: slideplayer.com
Source: slideplayer.com
A precipitation reaction is a reaction in which soluble ions in separate solutions are mixed together to form an insoluble compound that settles out of solution as a solid. The reaction is. Pesando luego este AgCl se determina la. It is a covalent nonelectrolyte b. 135 or in absolute ethanol.
 Source: youtube.com
Source: youtube.com
The compound sodium sulfate is soluble in water. Chemistry Notes for Students. It is soluble in water and in the dissolution process it ionizes. 15 CopperII hydroxide is a base but not an alkali. Alkalis Sodium hydroxide is an alkali because it dissolves in water to produce hydroxide ions.
Source:
Therefor it can dissolve other molecular solute only. C K2CO3 KI and KMnO4 are soluble. Dissolved in water water fills the box but is not shown. Solucionario quimica de raymond chang 12 edicion. When calcium hydroxide solution lime water reacts with carbon dioxide gas a white precipitate of calcium carbonate is formed along with water.
 Source: chegg.com
Source: chegg.com
Therefor it can dissolve other molecular solute only. During respiration glucose combines with oxygen in the cells of our body to form carbon dioxide and water along with the production of energy. Potassium dichromate is an oxidising agent in organic chemistry and is milder than potassium permanganate. 135 or in absolute ethanol. Compound Water Soluble Cation Anion a Na2CO3 yes 2.
 Source: chegg.com
Source: chegg.com
Commercial potassium sulfate contains not less than 48 soluble potash K2O chiefly as sulfate and not more than 25 chlorine FARM CHEM HDBK 1987 pB-57 Hazardous Substances Data Bank HSDB. A precipitation reaction is a reaction in which soluble ions in separate solutions are mixed together to form an insoluble compound that settles out of solution as a solid. Search thousands of other internships scholarships and other student programs in 120 countries. Free essays homework help flashcards research papers book reports term papers history science politics. Search Or if you are wondering who we are.
 Source: youtube.com
Source: youtube.com
NaOHaq Na aq OH aq An alkali is a soluble base which produces hydroxide ions OH aq in water. Potassium dichromate is an oxidising agent in organic chemistry and is milder than potassium permanganate. Plants readily absorb the K dissolved in the soil water. It is a covalent nonelectrolyte b. Identify the ions present in each compound then apply the rules to determine if the compound is soluble or insoluble.
 Source: clutchprep.com
Source: clutchprep.com
As soon as the soil waters K concentration drops additional. 14 If a base is soluble in water it is called an alkali. SECONDARY STAGE CHEMISTRY BOOK ONE FOR CLASS IX. Water is a molecule. The reaction is.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title k2so4 soluble or insoluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.