Is sodium iodide soluble
Home » chemistry » Is sodium iodide solubleIs sodium iodide soluble
Is Sodium Iodide Soluble. The precipitate lead chloride is insoluble in cold water but it is soluble in hot water. We use our combined professional expertise and team approach to provide our clients with the best services and products available. This is our newest publication and has been created to support the school technician profession in Scotland. Oxidation of Potassium Iodide by Hydrogen Peroxide.
 Sodium Iodide Wikipedia From en.wikipedia.org
Sodium Iodide Wikipedia From en.wikipedia.org
When an aqueous solution of sodium hydroxide NaOH is added to an aqueous solution of chromiumIII nitrate CrNO33 a precipitate of chromiumIII hydroxide CrOH3 forms. Make a slurry of 20 g soluble starch in 4 mL water. Write a balanced net ionic equation for this. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodine. Ksp value give a very clear clarification about precipitation. Sodium tungstate is the inorganic compound with the formula Na 2 WO 4.
The formula for lead iodide is PbI 2 and its molar mass is 46101 gramsmole.
But with lead 2 ion it forms lead chloride. IC-GREEN is to be administered intravenously. Salt is also used for countless other purposes such as removing. Drink the nutritional shake as a supplement snack or meal. PbI 2 - yellow. It is packaged with an Aqueous Solvent consisting of Sterile Water for Injection used to dissolve the indocyanine green.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sodium iodide is a water-soluble ionic compound with a crystal latticeSodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition but is more commonly used in veterinary medicine. They are white crystals which do not have an odour but possess a taste. Lead iodide must be stored to avoid contact with oxidizers such as perchlorates peroxides permanganates chlorates and nitrates and chemically active metals such as potassium sodium magnesium and zinc since violent reactions occur. Pour slurry into boiling water boil 5 minutes dilute to 200 mL allow to cool. Allow to stand for 2 - 3 minutes.
 Source: reddit.com
Source: reddit.com
Pour slurry into boiling water boil 5 minutes dilute to 200 mL allow to cool. This is our newest publication and has been created to support the school technician profession in Scotland. Pour slurry into boiling water boil 5 minutes dilute to 200 mL allow to cool. When an aqueous solution of sodium hydroxide NaOH is added to an aqueous solution of chromiumIII nitrate CrNO33 a precipitate of chromiumIII hydroxide CrOH3 forms. Idocyanine green is a water soluble tricarbocyanine dye with peak spectral absorption at 800 nm.

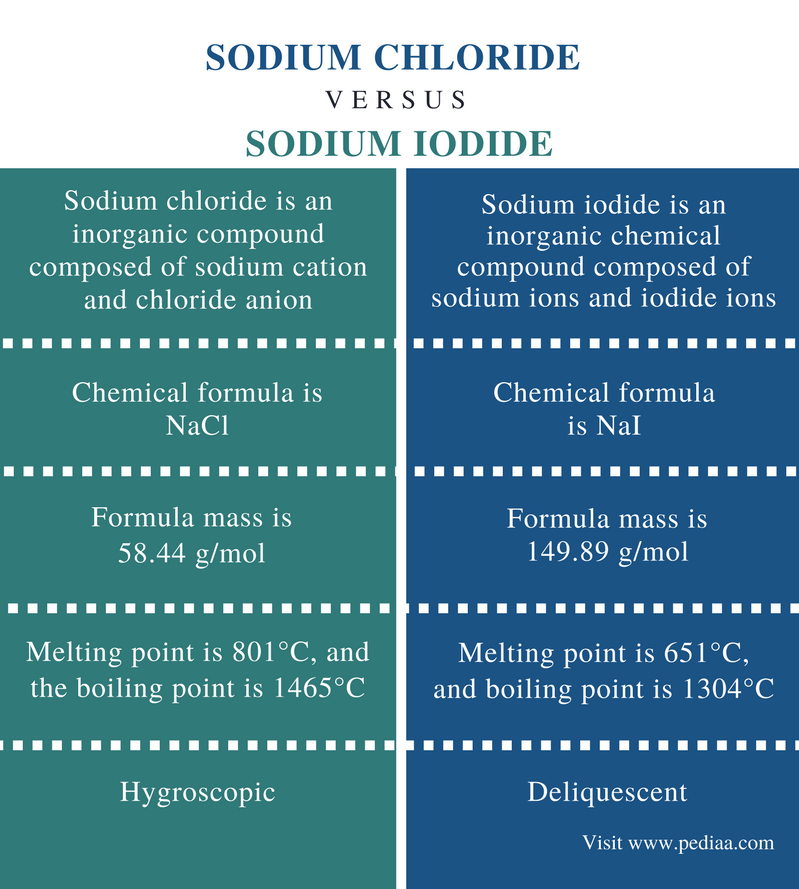
It has a melting point of 801C and a boiling point of 1413C. Silver chromate is sparingly soluble in aqueous solutions. Drink the nutritional shake as a supplement snack or meal. Identify halide ions in compounds - qualitative analysis Effect of ksp value for precipitation. It is an intermediate in the conversion of tungsten ores to the metal.
 Source: youtube.com
Source: youtube.com
From our locations in Pipestone Minnesota. The Ksp of Ag2CrO4 is 112 1012. Precipitates and colours of iodide ion. It is useful as a source of tungsten for chemical synthesis. In its aqueous state NaCl acts as a good conductor of electricity due to the free movement of the ions.
 Source: sodium.atomistry.com
Source: sodium.atomistry.com
Sodium iodide is a water-soluble ionic compound with a crystal latticeSodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition but is more commonly used in veterinary medicine. Continue the addition of the. Bring 100 mL distilled water to a boil. Make a slurry of 20 g soluble starch in 4 mL water. Idocyanine green is a water soluble tricarbocyanine dye with peak spectral absorption at 800 nm.
 Source: pediaa.com
Source: pediaa.com
In this case leadII nitrate PbNO_3_2 and sodium iodide NaI both. Sodium iodide is a water-soluble ionic compound with a crystal latticeSodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition but is more commonly used in veterinary medicine. Salt is the common name for the substance sodium chloride NaCI which occurs in the form of transparent cubic crystals. It has a melting point of 801C and a boiling point of 1413C. Ksp value give a very clear clarification about precipitation.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Youre dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution react to form an insoluble solid that precipitates out of solution. The Ksp of Ag2CrO4 is 112 1012. About Lead Iodide. A regulated marked area should be established where this chemical is handled use or stored. Add 2 mL of 5 NaOH solution and then introduce the potassium iodide - iodine reagent dropwise with shaking until a definite dark colour of iodine persists.

Stock solution 1 starch. Sodium iodide is a water-soluble ionic compound with a crystal latticeSodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition but is more commonly used in veterinary medicine. And Ottumwa Iowa we provide convenient services to our customers. The chemical name for Indocyanine Green is 1 H-Benzeindolium2-713-dihydro-11. Youre dealing with a double replacement reaction in which two soluble ionic compounds in aqueous solution react to form an insoluble solid that precipitates out of solution.
 Source: youtube.com
Source: youtube.com
They are white crystals which do not have an odour but possess a taste. The reaction equation for this chemical reaction is as follows. A regulated marked area should be established where this chemical is handled use or stored. AgI - yellow. It is useful as a source of tungsten for chemical synthesis.
 Source: byjus.com
Source: byjus.com
Glucerna Therapeutic Nutrition Shake For people with diabetes. Lead II iodide is a bright yellow solid that is slightly soluble in hot water. They are white crystals which do not have an odour but possess a taste. IC-GREEN is to be administered intravenously. Idocyanine green is a water soluble tricarbocyanine dye with peak spectral absorption at 800 nm.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is sodium iodide soluble by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.