Is sodium iodid soluble in water
Home » chemistry » Is sodium iodid soluble in waterIs sodium iodid soluble in water
Is Sodium Iodid Soluble In Water. Improvement in three cases of bulbar paralysis was so rapid that he considered iodine a virucidal agent. However because the benefits were forthcoming only after multiple doses he concluded that the iodide must have activated a defense mechanism. All sodium potassium and ammonium salts are soluble in water. Made by reacting a water-soluble lead compound with hydroiodic acid or with a soluble metal iodide.
 Sodium Iodide Wikipedia From en.wikipedia.org
Sodium Iodide Wikipedia From en.wikipedia.org
All sodium potassium and ammonium salts are soluble in water. It is readily purified by recrystallization from water. Federal Republic of Germany. One consequence of this is that sodium iodide is highly soluble in acetone whereas sodium chloride is not. Their time of arrival is measured with 20 ns precision. 50 pointsThe textarea shown to the left is named ta in a form named f1It contains the top 10000 passwords in order of frequency of use – each followed by a comma except the last one.
It is readily purified by recrystallization from water.
However iodine will form an aqueous solution in the presence of iodide ion such as by addition of potassium iodide KI because the triiodide ion is formed. The low solubility of silver iodide and lead iodide reflects the covalent character of these metal iodides. A test for the presence of iodide ions is the formation of yellow precipitates of these compounds upon treatment of a solution of silver nitrate or leadII nitrate. Federal Republic of Germany. Potassium Iodide is a metal halide composed of potassium and iodide with thyroid protecting and expectorant properties. 50 pointsThe textarea shown to the left is named ta in a form named f1It contains the top 10000 passwords in order of frequency of use – each followed by a comma except the last one.
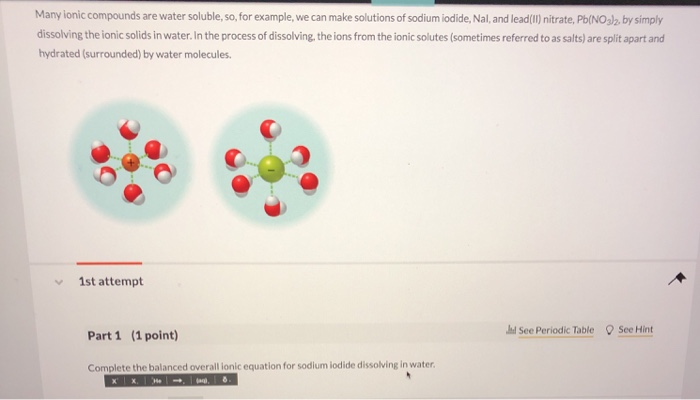 Source: chegg.com
Source: chegg.com
One consequence of this is that sodium iodide is highly soluble in acetone whereas sodium chloride is not. 2003 to Present p. All sodium potassium and ammonium salts are soluble in water. An icon used to represent a menu that can be toggled by interacting with this icon. 50 pointsThe textarea shown to the left is named ta in a form named f1It contains the top 10000 passwords in order of frequency of use – each followed by a comma except the last one.

50 pointsThe textarea shown to the left is named ta in a form named f1It contains the top 10000 passwords in order of frequency of use – each followed by a comma except the last one. Federal Republic of Germany. CCI Five Star - Test B. Improvement in three cases of bulbar paralysis was so rapid that he considered iodine a virucidal agent. When the Execute p1 button is clicked the javascript function p1 is executed.
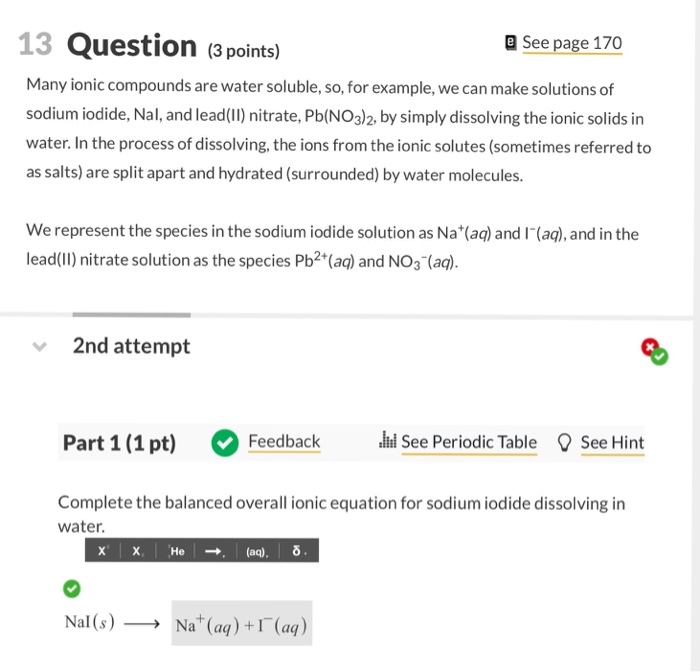
Edward himself used intravenous sodium iodide in doses of 500 to 900 mg dissolved in 10 ml of distilled water. Made by reacting a water-soluble lead compound with hydroiodic acid or with a soluble metal iodide. Ullmanns Encyclopedia of Industrial Chemistry. All sodium potassium and ammonium salts are soluble in water. Their time of arrival is measured with 20 ns precision.
 Source: en.wikipedia.org
Source: en.wikipedia.org
One consequence of this is that sodium iodide is highly soluble in acetone whereas sodium chloride is not. Potassium Iodide is a metal halide composed of potassium and iodide with thyroid protecting and expectorant properties. However because the benefits were forthcoming only after multiple doses he concluded that the iodide must have activated a defense mechanism. It is readily purified by recrystallization from water. Edward himself used intravenous sodium iodide in doses of 500 to 900 mg dissolved in 10 ml of distilled water.
 Source: youtube.com
Source: youtube.com
However iodine will form an aqueous solution in the presence of iodide ion such as by addition of potassium iodide KI because the triiodide ion is formed. However iodine will form an aqueous solution in the presence of iodide ion such as by addition of potassium iodide KI because the triiodide ion is formed. CCI Five Star - Test B. 50 pointsThe textarea shown to the left is named ta in a form named f1It contains the top 10000 passwords in order of frequency of use – each followed by a comma except the last one. A test for the presence of iodide ions is the formation of yellow precipitates of these compounds upon treatment of a solution of silver nitrate or leadII nitrate.
 Source: en.wikipedia.org
Source: en.wikipedia.org
2003 to Present p. Potassium iodide can block absorption of radioactive iodine by the thyroid gland through flooding the thyroid with non-radioactive iodine and preventing intake of radioactive molecules thereby protecting the thyroid from cancer causing radiation. Made by reacting a water-soluble lead compound with hydroiodic acid or with a soluble metal iodide. When the Execute p1 button is clicked the javascript function p1 is executed. Ullmanns Encyclopedia of Industrial Chemistry.
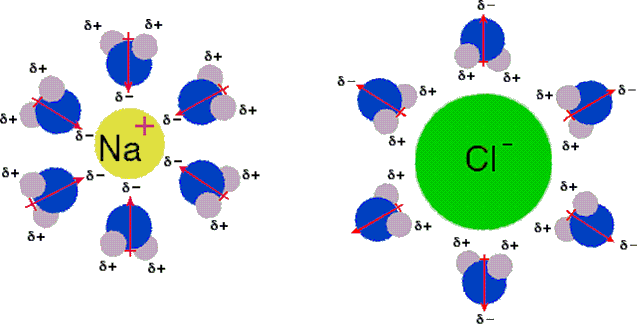 Source: reddit.com
Source: reddit.com
Improvement in three cases of bulbar paralysis was so rapid that he considered iodine a virucidal agent. 2003 to Present p. Potassium iodide can block absorption of radioactive iodine by the thyroid gland through flooding the thyroid with non-radioactive iodine and preventing intake of radioactive molecules thereby protecting the thyroid from cancer causing radiation. When the Execute p1 button is clicked the javascript function p1 is executed. Examples with Worked Solutions.
 Source: youtube.com
Source: youtube.com
Potassium iodide can block absorption of radioactive iodine by the thyroid gland through flooding the thyroid with non-radioactive iodine and preventing intake of radioactive molecules thereby protecting the thyroid from cancer causing radiation. The low solubility of silver iodide and lead iodide reflects the covalent character of these metal iodides. Fe forms FeF3 FeCl3 and FeBr3 but not FeI3. When the Execute p1 button is clicked the javascript function p1 is executed. Edward himself used intravenous sodium iodide in doses of 500 to 900 mg dissolved in 10 ml of distilled water.
 Source: sodium.atomistry.com
Source: sodium.atomistry.com
Improvement in three cases of bulbar paralysis was so rapid that he considered iodine a virucidal agent. One consequence of this is that sodium iodide is highly soluble in acetone whereas sodium chloride is not. A test for the presence of iodide ions is the formation of yellow precipitates of these compounds upon treatment of a solution of silver nitrate or leadII nitrate. Examples with Worked Solutions. Bromine is the third-lightest halogen and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas.
 Source: youtube.com
Source: youtube.com
2003 to Present p. Edward himself used intravenous sodium iodide in doses of 500 to 900 mg dissolved in 10 ml of distilled water. One consequence of this is that sodium iodide is highly soluble in acetone whereas sodium chloride is not. Improvement in three cases of bulbar paralysis was so rapid that he considered iodine a virucidal agent. An icon used to represent a menu that can be toggled by interacting with this icon.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is sodium iodid soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.