Is sodium carbonate soluble in water
Home » chemistry » Is sodium carbonate soluble in waterIs sodium carbonate soluble in water
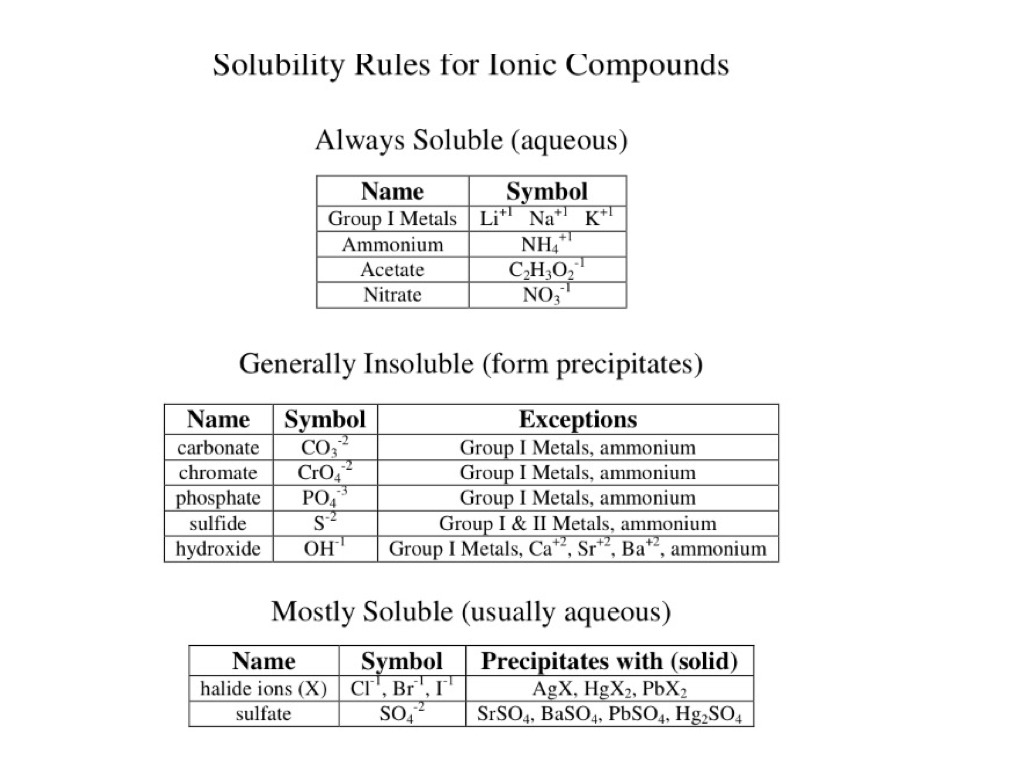
Is Sodium Carbonate Soluble In Water. Sodium hydroxide is used in several food processing applications such as curing foods like olives or helping. Sodium carbonate Na 2 CO 3 10H 2 O also known as washing soda soda ash and soda crystals is the inorganic compound with the formula Na 2 CO 3 and its various hydrates. Most multiple charged cations. At room temperature the threshold values are about 20 mglitre for sodium carbonate 150 mglitre for.
 Sodium Carbonate Wikipedia From en.wikipedia.org
Sodium Carbonate Wikipedia From en.wikipedia.org
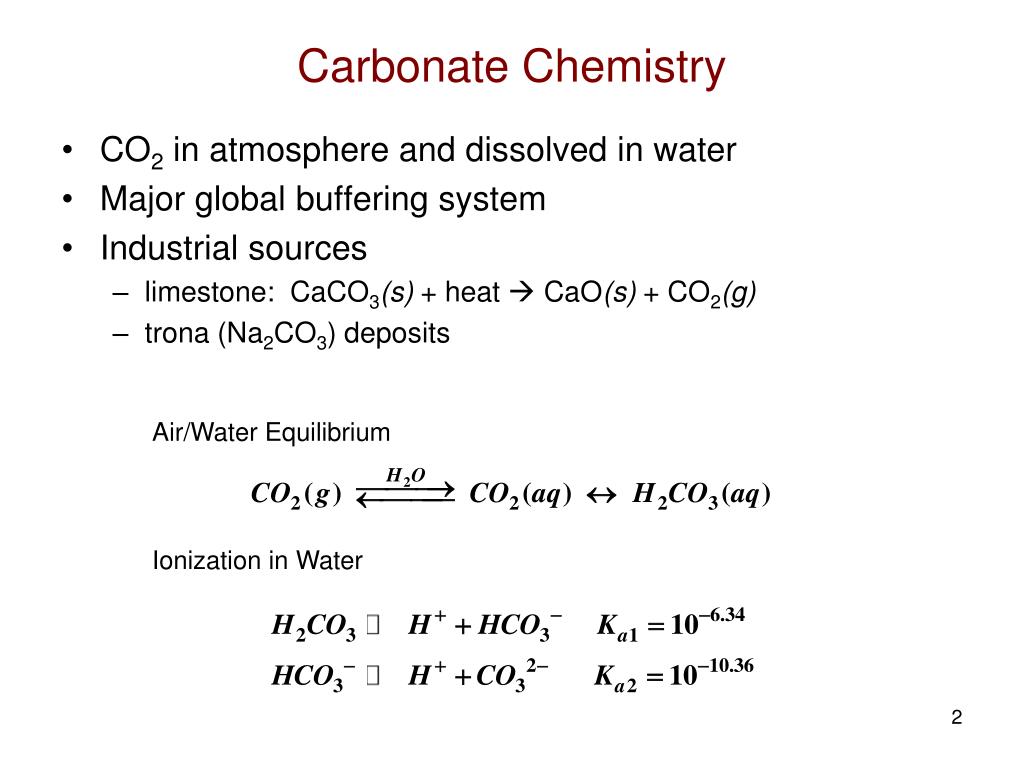
Sodium nitrite was developed during the 1960s. Dans le langage courant ce solide ionique le plus souvent sous forme poudreuse et ses solutions aqueuses sont dénommés. Sodium carbonate partially breaks down at high temperature to sodium hydroxide caustic and carbon dioxide. It is a strong base and acts as an antacid. Sodium silicate is a generic name for chemical compounds with the formula Na 2x Si y O 2yx or Na 2 O x SiO 2 y such as sodium metasilicate Na 2 SiO 3 sodium orthosilicate Na 4 SiO 4 and sodium pyrosilicate Na 6 Si 2 O 7The anions are often polymericThese compounds are generally colorless transparent solids or white powders and soluble in water in various amounts. Consequently calcium sulphate must be reacted upon chemically to cause a precipitate to form in the water where it can be.
A primary standard is a substance that can be used to make a standard solution directly.
Sodium hydroxide may be applied to prevent clogging of sewer pipes and sodium carbonate is applied in water purification to neutralize acids. From a health standpoint these minerals have no adverse effects and are in fact essential daily nutrients. Ca 2 aq CO 3. Both sodium and chloride are not rapidly depleted elements in a. Sodium Hydroxide in Water Treatment. Il sagit dun sel de sodium de lacide carbonique.
 Source: youtube.com
Source: youtube.com
Dans le langage courant ce solide ionique le plus souvent sous forme poudreuse et ses solutions aqueuses sont dénommés. Its boiling point is 101. Whether a water supply is labelled soft or hard is dependent on the presence of two highly soluble minerals calcium and magnesium. All forms are white odourless water-soluble salts that yield moderately alkaline solutions in water. Unlike competing products Equilibrium contains NO SODIUM CHLORIDE.
 Source: chegg.com
Source: chegg.com
In sanitary cleanser the element is present as sodium hypo chlorite. Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible. Its boiling point is 101. Water a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous liquid and solid states. These react with dissolved calcium ions forming a precipitate of calcium carbonate.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. It is also known as Soda crystals soda ash washing soda. When an ionic compound dissolves in water the solution contains ions rather than neutral. It is minerals that give water the refreshing flavor many people find desirable. It is an anionic surfactant as defined by the sulfate head group.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Sodium hypochlorite pentahydrate is a greenish yellow solid. It should take no more than 20 minutes to the point at. It is a strong base and acts as an antacid. At room temperature the threshold values are about 20 mglitre for sodium carbonate 150 mglitre for. It is an essential raw material in glass chemicals detergents and other important industrial products.
 Source: youtube.com
Source: youtube.com
A 01 M solution is made up. Water a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous liquid and solid states. When an ionic compound dissolves in water the solution contains ions rather than neutral. Sodium Hydroxide in Water Treatment. You can see the difference of values.
 Source: youtube.com
Source: youtube.com
It is minerals that give water the refreshing flavor many people find desirable. Alkanes alkyl halides and alkenes are not soluble in water. This means the sulfate head group shown by the pink shading in the diagram below is hydrophilic and water soluble while the 12-carbon-long chain is hydrophobic and water insoluble. Carboxylic acids react with NaHCO 3 to produce carbon dioxide bubbles as shown below in Equation 3. Sodium hypochlorite shows following physical and chemical properties Its molar mass is 744 gmol-1.
 Source: socratic.org
Source: socratic.org
Sodium carbonate Na 2 CO 3 10H 2 O also known as washing soda soda ash and soda crystals is the inorganic compound with the formula Na 2 CO 3 and its various hydrates. Sodium lauryl sulfate also is a widely used component of many nonpesticidal consumer products currently marketed in the. Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. It should take no more than 20 minutes to the point at. Most multiple charged cations.

Total Hardness of Water by EDTA Hardness is defined in terms of the capacity of cations in the water to replace the sodium or potassium ions in soaps and form sparingly soluble products insoluble. Sodium silicate is a generic name for chemical compounds with the formula Na 2x Si y O 2yx or Na 2 O x SiO 2 y such as sodium metasilicate Na 2 SiO 3 sodium orthosilicate Na 4 SiO 4 and sodium pyrosilicate Na 6 Si 2 O 7The anions are often polymericThese compounds are generally colorless transparent solids or white powders and soluble in water in various amounts. Containers must be tightly closed to prevent the conversion of NaOH to sodium carbonate by the CO2 in air. Whether a water supply is labelled soft or hard is dependent on the presence of two highly soluble minerals calcium and magnesium. These react with dissolved calcium ions forming a precipitate of calcium carbonate.
 Source: khanacademy.org
Source: khanacademy.org
Its melting point is 18. It should take no more than 20 minutes to the point at. At room temperature the threshold values are about 20 mglitre for sodium carbonate 150 mglitre for. Sodium lauryl sulfate also is a widely used component of many nonpesticidal consumer products currently marketed in the. Sodium hydroxide is also used to produce sodium hypochlorite a water disinfectant.
 Source: slideserve.com
Source: slideserve.com
However when calcium and magnesium permeate water they. Historically it was extracted from the ashes of plants growing in sodium-rich soils. Il sagit dun sel de sodium de lacide carbonique. At room temperature the threshold values are about 20 mglitre for sodium carbonate 150 mglitre for. Most multiple charged cations.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is sodium carbonate soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.