Is silver phosphate soluble in water
Home » chemistry » Is silver phosphate soluble in waterIs silver phosphate soluble in water
Is Silver Phosphate Soluble In Water. Which if any silver is soluble. 55 77 FAP has two main advantages over HA and fluorine is often added to drinking water to encourage the conversion. Supernatant was removed and the pellet washed 3 hydrogenoformans was highly soluble in water the pellet obtained by times in MilliQ water to remove any remaining of the medium. Phosphoric acid is simply a phosphorus atom shown as the P in the line drawing to the left with 4 oxygen atoms bonded to it.
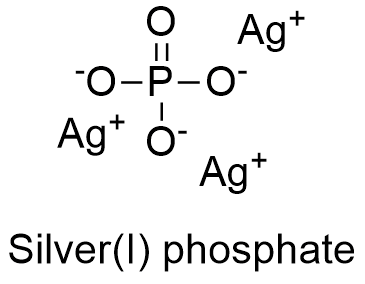 Silver Phosphate Formula From softschools.com
Silver Phosphate Formula From softschools.com
But with lead 2 ion it forms lead. Silver phosphate Ag 3 PO 4 is yellow precipitate and dissolve in dilute nitric acid and ammonia. The water solubility of some iron compounds increases at lower pH values. Ag ClO3 see rule 3 f. Lead compounds are generally soluble in soft slightly. Its maximum solubility is 30kg of Urea per 100 litres of water.
But with lead 2 ion it forms lead.
A well-known example of a water soluble lead compound is lead sugar leadIIacetate which derived its name from its sweet nature. Be aware that when Urea is mixed with water this creates a cold endothermic reaction so take care on cold frosty mornings. Calcium phosphate stone crystals form when calcium atoms combine with phosphoric instead of oxalic acid and produce the calcium phosphate kidney stone. Water prior to any characterization. Ag 2 CO 3. Ag 2 CrO 4.
 Source: youtube.com
Source: youtube.com
A Compounds are more soluble in acidic solutions because they break down in water. Solubleaq insoluble s watch for the 5 exceptionsLINK to Sol. Over-enrichment of phosphate in both fresh and inshore marine waters can lead to massive algae blooms. Coli 300000 Container freight depot operators. Lead compounds are generally soluble in soft slightly.
 Source: clutchprep.com
Source: clutchprep.com
If a product is soluble aq in line 2 write the ions that make it up under it with between them. Heavy metals such as silver mercury and lead form salts having more covalent character 3rd example and the water solubility is reduced especially for acids composed of four or more. Acidic solutions have stronger acids that increase solubility than in pure water. Water prior to any characterization. When you drop an ionic compound in water these water magnets will gather around it trying to pull the positive and negative ions apart.
 Source: clutchprep.com
Source: clutchprep.com
It is an excellent groundcover and complements a soothing silvery color palette. Ag 2 CrO 4. Similar to iron ore. Some ionic compounds arent stuck together very well. Just write them as they appear on line 2 keep them on the right side of the arrow 8.
 Source: slideplayer.com
Source: slideplayer.com
Heavy metals such as silver mercury and lead form salts having more covalent character 3rd example and the water solubility is reduced especially for acids composed of four or more. Na OH see rule 1 d. 103 128 This can help to counteract any demineralization. Immense quantities of phosphate rock or phosphorite occur in sedimentary shelf deposits ranging in age from the Proterozoic to currently forming environments. Ferric phosphate - FePO 4 - yellow precipitate - dissolve in HNO 3 and not dissolve in CH 3 COOH.
 Source: youtube.com
Source: youtube.com
103 128 This can help to counteract any demineralization. Primary Source Water Type. Recall from the solubility rules in an earlier chapter that halides of Ag are not normally soluble. One end has a positive charge while the other has a negative. SEM-EDX analysis of two areas from a calcinated.
 Source: youtube.com
Source: youtube.com
Other iron compounds may be more water soluble than the examples mentioned above. If silver nitrate is involved in a fire flood with water from as far away as possible do not use dry chemical CO2 or Halon. Supernatant was removed and the pellet washed 3 hydrogenoformans was highly soluble in water the pellet obtained by times in MilliQ water to remove any remaining of the medium. If a product is soluble aq in line 2 write the ions that make it up under it with between them. SEM-EDX analysis of two areas from a calcinated.
 Source: chegg.com
Source: chegg.com
Water prior to any characterization. Phosphate is used in fertilisers. Here are the main reasons for solubility. Acetate carbonate phosphate and sulfide. Select the compounds that are always soluble in water bolded are correct a.
 Source: youtube.com
Source: youtube.com
Soluble in water ethanol and acetone. Generally the quality degradation of carp fillets was remarkably alleviated using coatings when compared to the control. It is an excellent groundcover and complements a soothing silvery color palette. One end has a positive charge while the other has a negative. 55 77 FAP has two main advantages over HA and fluorine is often added to drinking water to encourage the conversion.
 Source: chegg.com
Source: chegg.com
Chloride ion with sodium ion form sodium chloride which is highly soluble in water. Some ionic compounds arent stuck together very well. Ag 2 CO 3. When you drop an ionic compound in water these water magnets will gather around it trying to pull the positive and negative ions apart. Water molecules H 2 O have an unusual structure which makes them similar to a magnet.
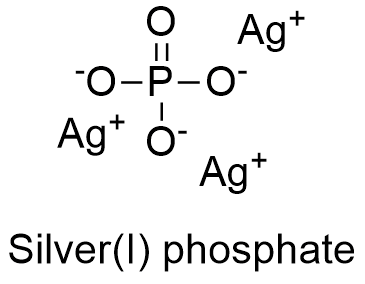 Source: softschools.com
Source: softschools.com
Ag 2 CrO 4. We would like to show you a description here but the site wont allow us. HG2I2 see rule 3 c. 103 128 This can help to counteract any demineralization. A well-known example of a water soluble lead compound is lead sugar leadIIacetate which derived its name from its sweet nature.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is silver phosphate soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.