Is nickel chloride soluble
Home » chemistry » Is nickel chloride solubleIs nickel chloride soluble
Is Nickel Chloride Soluble. Its important to remember that common names are inaccurate and vary from one place and time to another. Prolonged exposure to fire or heat may result in an explosion. This compound is liable to explode when mixed with dinitrogen pentaoxide or nitric acid. Subject to slow hydrolysis which is accelerated by light.
Nickel Ii Chloride Cl2ni Chemspider From chemspider.com
Cadmium has only. At a pH of 80 nickel has a solubility of 70 mgl and at a pH of 102 the solubility is 01 mgl. Fe and other metals. Nickel chloride and nickel sulfide may also induce both histone ubiquitination and phosphorylation in A459 cells 9496. Furthermore following the addition of small amounts of sodium chloride or ethanol to the solutions to slightly alter the chemical interactions the machine learning algorithm could differentiate polymers with similar properties. Treatment of A459 cells with soluble or insoluble nickel compounds resulted in increased levels of H2A and H2B ubiquitination in a dose- and time-dependent manner indicating that both soluble and insoluble nickel compounds.
Thus if K sp Mm An is the equilibrium constant for the reaction M m A n s mMaq nA aq where M m A n is the slightly.
The total nickel ion concentration is a. Miscellaneous applications of nickel oxide include its use in. Class nickel inorganic Nickel carbonyl Nickel sulfide roasting 4-Nitrobiphenyl p-Nitrochlorobenzene 2-Nitronaphthalene 2-Nitropropane N-Nitrosodimethylamine. NickelII chloride or just nickel chloride is the chemical compound NiCl 2. Chromium reaches its least theoretical chromium solubility of 008 at pH of 75. B the manufacture of nickel salts eg chloride nitrate and sulfate which can be used to make refined nickel oxide.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The Gravimetric Determination of Nickel INTRODUCTION NickelII forms a precipitate with the organic compound. Methylene chloride 44-Methylenedianiline MDA N. This is a list of archaic chemical names and common names for chemicals with their modern. The Gravimetric Determination of Nickel INTRODUCTION NickelII forms a precipitate with the organic compound. Thus if K sp Mm An is the equilibrium constant for the reaction M m A n s mMaq nA aq where M m A n is the slightly.
 Source: sciencedirect.com
Source: sciencedirect.com
It occurs mainly in association with the sulfide ores of zinc lead and copper. Miscellaneous applications of nickel oxide include its use in. Nickel nitrate is a green crystalline solid. Nickel sensitivity is observed to be three to five times more frequent in women than in men. Nickel salts have been shown to be.
Source: chemspider.com
Lastly the new peptide sensor and algorithm were tested by the researchers on actual wastewater and the potential to detect various water-soluble polymers was. 1 H2O takes care of the 2H and one of the O atoms 1 CO2 consumes the 1 C and the remaining 2 O atoms. Nickel has a similar curve but it occurs at 3 pH points high. Mixtures of this compound in air with methanol. Nickel carbonyl IUPAC name.
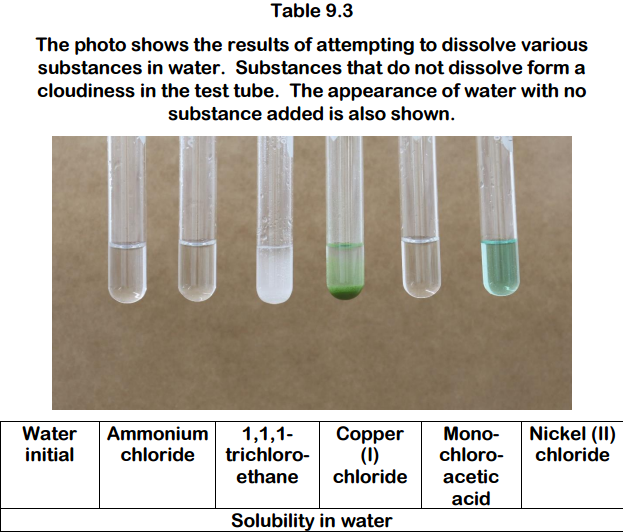 Source: bartleby.com
Source: bartleby.com
Ammonium Chloride NH 4 Cl- Ammonium chloride is an inorganic compound with formula NH 4 Cl. NickelII nitrate carbon dioxide and water. Treatment of A459 cells with soluble or insoluble nickel compounds resulted in increased levels of H2A and H2B ubiquitination in a dose- and time-dependent manner indicating that both soluble and insoluble nickel compounds. Methylene chloride 44-Methylenedianiline MDA N. NickelII carbonate and nitric acid Products.
 Source: researchgate.net
Source: researchgate.net
NickelII chloride or just nickel chloride is the chemical compound NiCl 2. Methylene chloride 44-Methylenedianiline MDA N. Treatment of A459 cells with soluble or insoluble nickel compounds resulted in increased levels of H2A and H2B ubiquitination in a dose- and time-dependent manner indicating that both soluble and insoluble nickel compounds. Lastly the new peptide sensor and algorithm were tested by the researchers on actual wastewater and the potential to detect various water-soluble polymers was. NickelII carbonate and nitric acid Products.
 Source: bengislife.com
Source: bengislife.com
NickelII chloride or just nickel chloride is the chemical compound NiCl 2. Recall from the solubility rules in an earlier chapter that halides of Ag are not normally soluble. Nickel nitrate is a green crystalline solid. Subject to slow hydrolysis which is accelerated by light. Thus if K sp Mm An is the equilibrium constant for the reaction M m A n s mMaq nA aq where M m A n is the slightly.
 Source: youtube.com
Source: youtube.com
Miscellaneous applications of nickel oxide include its use in. Its important to remember that common names are inaccurate and vary from one place and time to another. NickelII chloride or just nickel chloride is the chemical compound NiCl 2. 1 H2O takes care of the 2H and one of the O atoms 1 CO2 consumes the 1 C and the remaining 2 O atoms. Calculating the aqueous solubility of sparingly soluble compounds under various conditions.
 Source: en.wikipedia.org
Source: en.wikipedia.org
NickelII nitrate carbon dioxide and water. The anhydrous salt is yellow but the more familiar hydrate NiCl 2 6H 2 O is green. Several inorganic cadmium compounds are quite soluble in water eg. Because precipitate is in the solid phase and deposited at bottom of the solution. It may be determined by direct measure- ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is noncombustible but it will accelerate the burning of combustible materials. Its important to remember that common names are inaccurate and vary from one place and time to another. These anions selectively form tightly bound soluble complexes with the metals and prevent the formation of insoluble metal hydroxides in the buffered solution. Nickel sensitivity is observed to be three to five times more frequent in women than in men. Several inorganic cadmium compounds are quite soluble in water eg.
 Source: sciencedirect.com
Source: sciencedirect.com
Methylene chloride 44-Methylenedianiline MDA N. Nickel salts have been shown to be. Toxic oxides of. NickelII chloride in various forms is the most important source of nickel for chemical synthesis. It may be determined by direct measure- ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is nickel chloride soluble by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.