Is naoh soluble in water
Home » chemistry » Is naoh soluble in waterIs naoh soluble in water
Is Naoh Soluble In Water. All soluble hydroxides like lithium cesium sodium potassium etc. A pH higher than 70 at standard conditions. Alkalis are soluble bases. Sodium hydroxide NaOH also known as caustic soda or lye is a highly versatile substance used in a variety of manufacturing processes.
 Solved 1 Solid Sodium Hydroxide Dissolves In Water To Form Chegg Com From chegg.com
Solved 1 Solid Sodium Hydroxide Dissolves In Water To Form Chegg Com From chegg.com
Sodium hydroxide is less soluble in polar solvents such as methanol but is readily soluble in water up to a 50 wt solution which will have a pH around 14. The water treatment facilities purify and deaerate make-up water or feed water. The only way to avoid ASR in the presence of siliceous aggregates and water is to maintain the concentration of soluble alkali NaOH and KOH at the lowest possible level in concrete so that the catalysis mechanism becomes negligible. Simple things like running all the taps ensures good water quality and refills any sink traps. In case of contact immediately flush eyes with plenty of water for a t least 15 minutes. Impure soluble salts can be purified by using recrystallisation.
NaOH is a one normal solution meaning that one mole of sodium hydroxide liberates one OH- molecule for neutralization.
Besides water substances that can donate and accept protons like the bicarbonate ion are amphoteric. Lets solve a problem about FeCl 3 NaOH reaction. Sodium sulphate is highly soluble and remains in solution unless the water is evaporated almost to dryness. The formation of benzoic acid has to do with ionizability Water can attach to benzoic acid by hydrogen bonding. This produces a water-soluble pleasant-smelling white powder that is used for flavorings and perfume. Sodium hydroxide is less soluble in polar solvents such as methanol but is readily soluble in water up to a 50 wt solution which will have a pH around 14.
 Source: youtube.com
Source: youtube.com
Crystalline Al2O3 at room temperature is hardly soluble in any solvent even concentrated acid or alkali for kinetic reasons. Sometimes sodium hydroxide is. Therefore it exists as an aqueous solution and NaOH also exists as a solution. A soluble base is also called an alkali. The alkali-silica reaction mechanism catalysed by a soluble strong base as NaOH or KOH in the presence.
 Source: chegg.com
Source: chegg.com
A pH higher than 70 at standard conditions. The alkali-silica reaction mechanism catalysed by a soluble strong base as NaOH or KOH in the presence. Solubility Rules for Ionic Compounds in Water. Adds water carbon dioxide or ammonia across double bonds or eliminate these to create double bonds. Therefore it exists as an aqueous solution and NaOH also exists as a solution.
Source: quora.com
A salt is soluble if it dissolves in water to give a solution with a concentration of at least 01 moles per liter at room temperature. NaOH is bitter and has a soapy feel to it. Impure soluble salts can be purified by using recrystallisation. GL345 Guidance for science departments returning to school after an extended period of closure. In case of contact immediately flush eyes with plenty of water for a t least 15 minutes.
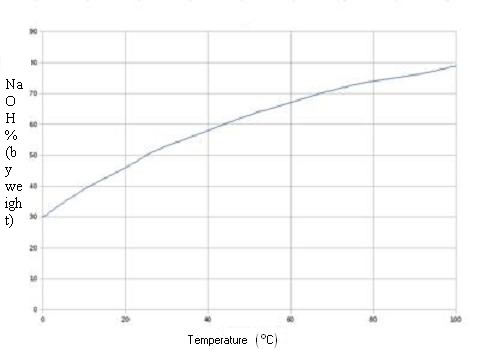 Source: hydro-land.com
Source: hydro-land.com
Besides water substances that can donate and accept protons like the bicarbonate ion are amphoteric. A reaction between an acid and a base is called neutralization and this neutralization results in. Lets solve a problem about FeCl 3 NaOH reaction. The Na K. Sodium Hydroxide in Food Production.
Source: socratic.org
A reaction between an acid and a base is called neutralization and this neutralization results in. Sodium hydroxide NaOH also known as caustic soda or lye is a highly versatile substance used in a variety of manufacturing processes. The Isomerases enzymes catalyze the structural shifts present in a molecule thus causing the change in the shape of the molecule. The chemical solution of 50 NaOH is denser than water with a density around 152gcm 3 at 68F. Recrystallisation can be repeated many times to obtain salt crystals which are very pure.
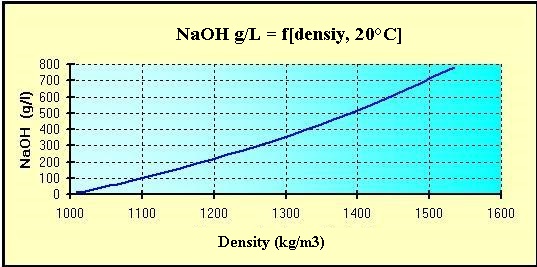 Source: hydro-land.com
Source: hydro-land.com
NaOH is bitter and has a soapy feel to it. Simple things like running all the taps ensures good water quality and refills any sink traps. Sodium hydroxide is also used to produce sodium hypochlorite a water disinfectant. Correspondingly a base was any compound that when dissolved in water gives a solution with a hydrogen ion activity lower than that of pure water ie. Adds water carbon dioxide or ammonia across double bonds or eliminate these to create double bonds.
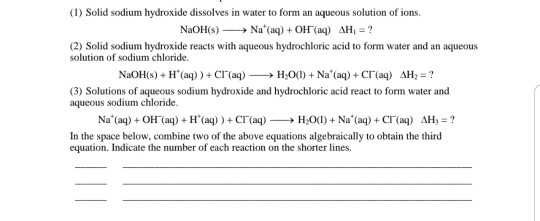 Source: chegg.com
Source: chegg.com
Water and Aqueous Solutions. A salt is soluble if it dissolves in water to give a solution with a concentration of at least 01 moles per liter at room temperature. Correspondingly a base was any compound that when dissolved in water gives a solution with a hydrogen ion activity lower than that of pure water ie. Sometimes sodium hydroxide is. In case of contact immediately flush eyes with plenty of water for a t least 15 minutes.
 Source: quora.com
Source: quora.com
The Na K. Soluble - soluble more than 1g per 100g of water low - low solubility 001g to 1g per 100g of water insoluble - insoluble less than 001g per 100g of water not exist - do not exist in the aqueous environment. A pH higher than 70 at standard conditions. Alkalis are soluble bases. Unlike chemicals such as lime NaOH is very highly soluble and relatively easy to handle.
 Source: youtube.com
Source: youtube.com
Therefore it exists as an aqueous solution and NaOH also exists as a solution. Therefore it exists as an aqueous solution and NaOH also exists as a solution. Solubility Rules for Ionic Compounds in Water. Correspondingly a base was any compound that when dissolved in water gives a solution with a hydrogen ion activity lower than that of pure water ie. FeCl 3 is soluble in water.
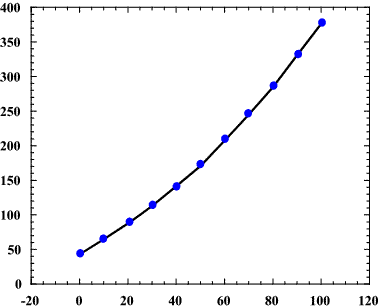 Source: socratic.org
Source: socratic.org
A reaction between an acid and a base is called neutralization and this neutralization results in. Specific GravityDensity213 gcm3 Molecular FormulaNaOH Molecular Weight40 Section 10 - Stability and Reactivity Chemical Stability. Solubility Rules for Ionic Compounds in Water. Note that while calcium hydroxide barium hydroxide and strontium hydroxide are strong bases they are not very soluble in water. A salt is insoluble if the concentration of an aqueous solution is less than 0001 M at room temperature.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is naoh soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.