Is lead chlorate soluble
Home » chemistry » Is lead chlorate solubleIs lead chlorate soluble
Is Lead Chlorate Soluble. This distinguishable property helps identify acids from other compounds such as salt and bases. Uses of different salts in daily life Many different types of salts can be found in nature. If you look. All ammonium potassium and sodium salts are soluble.

Potassium chlorate and sugar powder creates a huge amount of fouling even more than normal BP. The chloride bromide and iodide ions almost always make soluble compounds called halogen salts. A solidsolid reaction between lead nitrate and potassium iodide. Which lead II compound is water soluble. Tips for Success Predicting a Precipitate. General rules for the solubility of salts.
Pay particular attention to compounds listed as slightly soluble and remember that temperature affects solubility.
Which of the following ions always form soluble ionic compounds_ select all that apply. In association with Nuffield Foundation. There are many better ways to approach. Also adding vaseline to shitty black powder substitute doesnt magically make it plastic explosive. And barite is used in paints bricks tiles glass and rubber production. The chloride bromide and iodide ions almost always make soluble compounds called halogen salts.
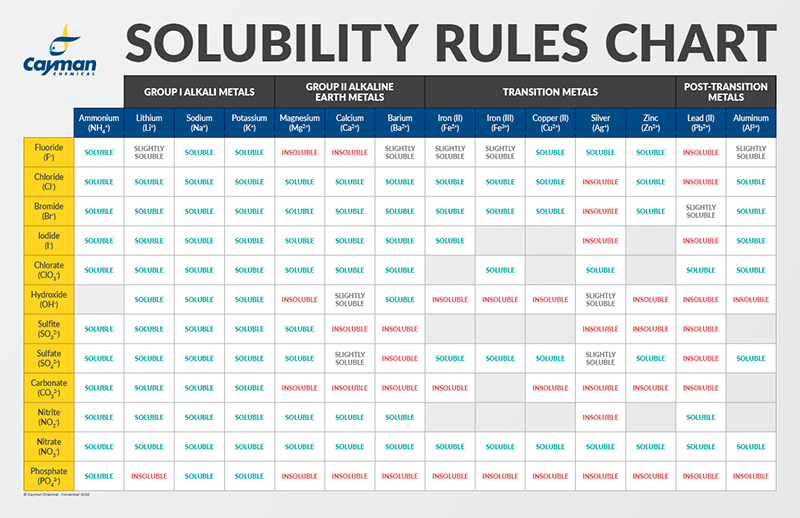 Source: caymanchem.com
Source: caymanchem.com
But what is more remarkable yet this chloride of pottasium itself has like peroxide of manganese. B the polarity of the solute and solvent must be opposite. The oxygen had to have been not very water soluble in order for it to displace the water in the florence flask. Ammonium NH 4 potassium K sodium Na. Bromides Br chlorides Cl and iodides I.
 Source: qcpages.qc.cuny.edu
Source: qcpages.qc.cuny.edu
A NH3 B NaCl C CH3OH D N2. All ammonium potassium and sodium salts are soluble. The chloride bromide and iodide ions almost always make soluble compounds called halogen salts. If you look. Many acids can be hazardous if ingested and shouldnt be tasted.
 Source: slideplayer.com
Source: slideplayer.com
Stability and Reactivity Data. Once the acid binds to the base it becomes a neutral substance. A simple demonstration of catalysis also introducing the idea of an activated complex and to. If the following ions Ca 2 Cl - Na and CO 3 2- are placed in a test tube the precipitate formed is _____. It is appreciably soluble in water and heavier so may be expected to sink and dissolve at a rapid rate.
 Source: quizlet.com
Source: quizlet.com
25 C 29815 K. Once the acid binds to the base it becomes a neutral substance. D the solute-solvent forces must be. Metal hydroxides and oxides. Insoluble in cold water.
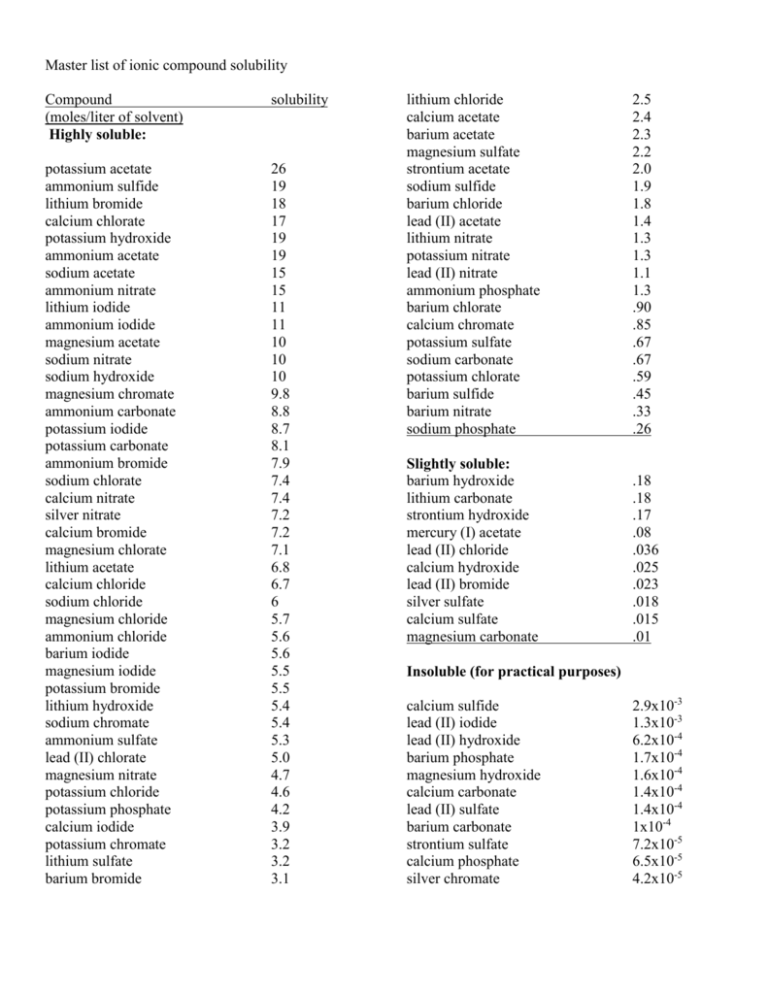 Source: studylib.net
Source: studylib.net
25 C 29815 K. By warming pure chlorate of pottasium we obtain pure oxygen but the presence of the smallest quantity of chloride of pottasium is sufficient to change part of the oxygen into ozone. It is appreciably soluble in water and heavier so may be expected to sink and dissolve at a rapid rate. Most bromides are solubleExceptions. A the solute-solute forces must be greater than the solute-solvent forces.
 Source: sciencenotes.org
Source: sciencenotes.org
Insoluble in chromium trioxides and mineral acids alkalies. Potassium chlorate and sugar powder creates a huge amount of fouling even more than normal BP. Salts containing silver lead and mercury. What is Infobox references. Any box that reads soluble results in an aqueous product in which no precipitate has.

A visible activated complex. Sulfides are one of the most insoluble ions. A solubility chart is a chart with a list of ions and how when mixed with other ions they can become precipitates or remain aqueous. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. For example a solution of calcium chloride is typically considered soluble in water yet if the water is cold enough the salt doesnt readily dissolve.
 Source: youtube.com
Source: youtube.com
The product is stable. Reaction with NOM lead to the formation of by-products such as chlorite ClO 2 a compound that is of health concern. D the solute-solvent forces must be. A CaCO3 b NaCl c CaCl2 d no precipitate will be formed. Many acids can be hazardous if ingested and shouldnt be tasted.

In giving rise to this development of ozone the choride of pottasium remains itself completely unaltered. Ozone is known to react both. The oxygen had to have been not very water soluble in order for it to displace the water in the florence flask. Sulfides are one of the most insoluble ions. B the polarity of the solute and solvent must be opposite.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
This distinguishable property helps identify acids from other compounds such as salt and bases. By warming pure chlorate of pottasium we obtain pure oxygen but the presence of the smallest quantity of chloride of pottasium is sufficient to change part of the oxygen into ozone. Calcium compounds are more or less water soluble. In order for a solute to dissolve in solution. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is lead chlorate soluble by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.