Is cuso4 soluble or insoluble in water
Home » chemistry » Is cuso4 soluble or insoluble in waterIs cuso4 soluble or insoluble in water
Is Cuso4 Soluble Or Insoluble In Water. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. As all of the other substances are soluble in water we can rewrite the equation. Which one of the following compounds will NOT be soluble in water. The pentahydrate CuSO 4 5H 2 O the most commonly encountered salt is bright blue.

What is wrong with this word equation calcium chloride plus magnesium sulfate. Is BaCl2 a precipitate. View Answer Sparkling wine is bottled under a CO_2. Compound Cation Anion a KOH K OH- b K2SO4 2 K SO42- c LiNO3 Li NO3- d NH42SO4 2 NH4 SO42- 15. CHEM 103 Exam 1-6 Final - Complete Solutions Graded A Exam 1-6 Question 1 1. In the presence of a lipid rich solution and water Sudan III will diffuse through the solution producing an orange-pink color.
Method 2 of 2.
Clean the steel a second time with isopropyl alcohol. The odor is not clinically significant. Write the reaction and identify the precipitate. In Alkaline urine amorphous phosphate and carbonates are seen. NH43PO4 Mg3PO42 Ca3PO42 AlPO4 Cu3PO4 NH43PO4. You can either draw a freehand image or replicate an existing image onto the steel surface.

Chapter 10 Acids Bases and Salts 15. The odor is not clinically significant. Answer 1 of 5. Li2O Na2CO3 two of these would not be soluble NH4C2H3O2 CaCO3 Based on the solubility rules will the following reaction occur because a precipitate will form. View Answer Sparkling wine is bottled under a CO_2.
 Source: tes.com
Source: tes.com
Compound Cation Anion a KOH K OH- b K2SO4 2 K SO42- c LiNO3 Li NO3- d NH42SO4 2 NH4 SO42- 15. Which of the following statements is correct about an aqueous solution of an acid and of a base. Barium Chloride is soluble in water and Barium Sulfate is insoluble in water. 1 ft 12 inches 1 pound 16 oz 1 gallon 4 quarts 1 mile 5280 feet 1 ton 2000 pounds 1. Which one of the following statements is are correct.

This is not part. Soluble in ether. Rinse the steel with water. It was observed that there was an increase in the temperature of the solution contained in beakers A and B whereas in case of beaker C the temperature of the solution falls. Write the reaction and identify the precipitate.
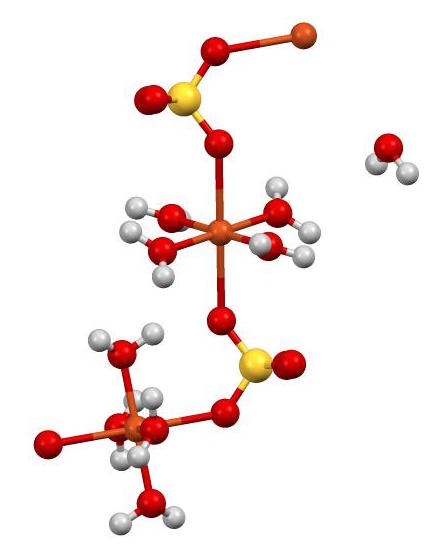 Source: en.wikipedia.org
Source: en.wikipedia.org
In Alkaline urine amorphous phosphate and carbonates are seen. While halides are generally soluble those of Ag are not. It was observed that there was an increase in the temperature of the solution contained in beakers A and B whereas in case of beaker C the temperature of the solution falls. The reason for this is. A small amount of NaOH anhydrous CuSO4 and NaCl were added to the beakers A B and C respectively.
 Source: youtube.com
Source: youtube.com
Free WAD and total cyanides will all react with ferrous iron to yield a variety of soluble and insoluble compounds primarily hexacyanoferrate III FeCN3- Prussian blue FeFeCN and other insoluble metal-iron-cyanide MFeCN6 compounds such as those with copper or zinc Adams 1992. Free WAD and total cyanides will all react with ferrous iron to yield a variety of soluble and insoluble compounds primarily hexacyanoferrate III FeCN3- Prussian blue FeFeCN and other insoluble metal-iron-cyanide MFeCN6 compounds such as those with copper or zinc Adams 1992. Question 2 Using the following information do the conversions shown below showing all work. Which one of the following compounds will NOT be soluble in water. Soluble in methanol 11 g100 ml but insoluble in ethanol.
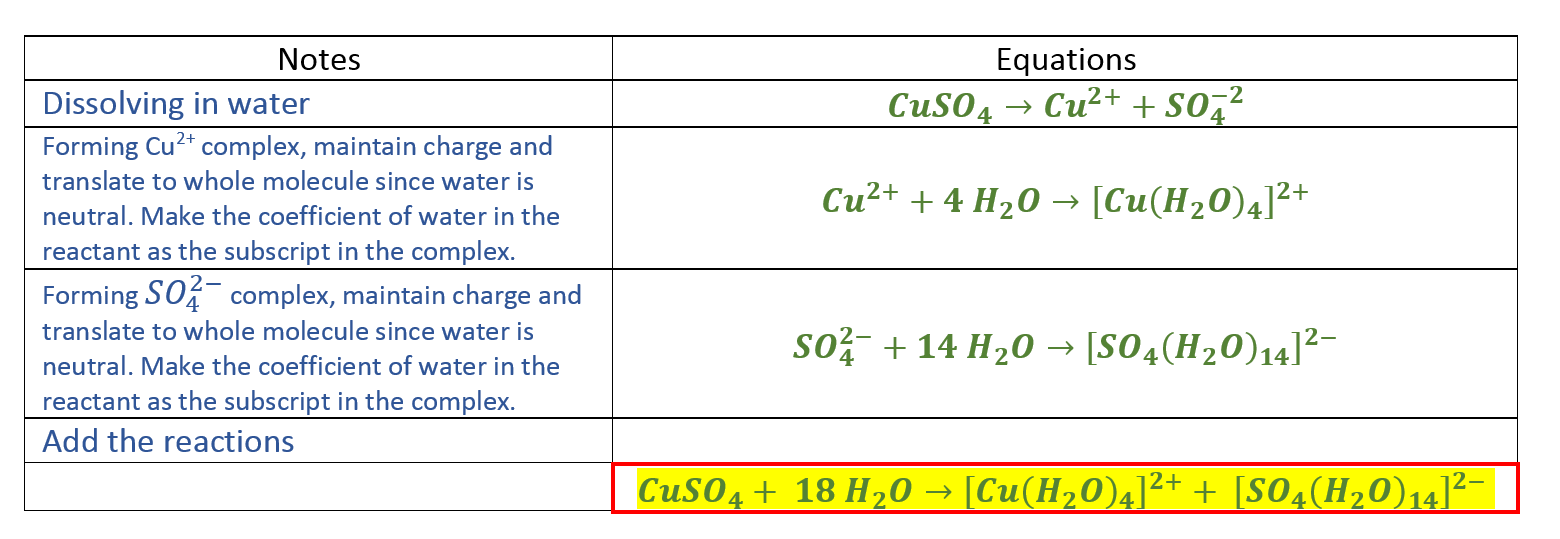 Source: clutchprep.com
Source: clutchprep.com
However distilled water cannot be an electrolyte. Soluble in ether. Soluble by heat are amorphous urates and uric acid crystals. Salts are generally insoluble. Choose the image you want to etch into the steel.
 Source: youtube.com
Source: youtube.com
Soluble by heat are amorphous urates and uric acid crystals. Answer 1 of 5. What is wrong with this word equation calcium chloride plus magnesium sulfate. Which of the following statements is correct about an aqueous solution of an acid and of a base. Is BaCl2 a precipitate.
 Source: youtube.com
Source: youtube.com
An aqueous solution of potassium chloride is mixed with an aqueous solution of sodium nitrate. Which of the following ionic compounds is soluble in water. NH43PO4 Mg3PO42 Ca3PO42 AlPO4 Cu3PO4 NH43PO4. Li2O Na2CO3 two of these would not be soluble NH4C2H3O2 CaCO3 Based on the solubility rules will the following reaction occur because a precipitate will form. Ii They have high melting points and boiling points.
 Source: slideplayer.com
Source: slideplayer.com
Spermatozoa bacteria yeast and WBCs. The saturation temperature for 207 g of CuSO4 soluble in water is. Which of the following statements is correct about an aqueous solution of an acid and of a base. Barium chloride and potassium sulfate are both ionic compounds. This happens as the carbon dioxide forms acidic carbonic acid when it dissolves in the water the carbonic acid H2CO3 reacts further with the calcium carbonate.
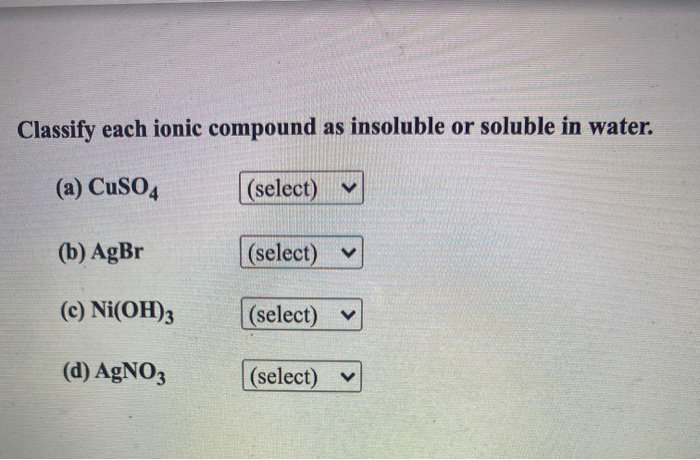 Source: chegg.com
Source: chegg.com
In Alkaline urine amorphous phosphate and carbonates are seen. Solid iron is placed in a solution of lead ii nitrate. Question 2 Using the following information do the conversions shown below showing all work. Zn CuSO4 ZnSO4 Cu Iron displaces copper from copper sulphate solution Fe CuSO4 FeSO4 Cu after 15 20 minutes. Lipids chyle and lymphatic fluid.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is cuso4 soluble or insoluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.