Is cuso4 soluble in water
Home » chemistry » Is cuso4 soluble in waterIs cuso4 soluble in water
Is Cuso4 Soluble In Water. What happens when zinc reacts with copper. A typical example of a single displacement reaction where one metal displaces another is the reaction between iron and copper sulfate given by the reaction Fe CuSO 4 FeSO 4 Cu. A displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. NH43PO4 Mg3PO42 Ca3PO42 AlPO4 Cu3PO4 NH43PO4.
What Happens When Cuso4 Dissolves In Excess Kcn Quora From quora.com
It is used in fertilizers and in processed food. 10 mgml bovine serum albumin BSA Method 1. 2 NaBraq PbNO32aq– 2 NaNO3 PbBr2 yes NaNO3 is insoluble more information is required No both products are soluble yes both products are insoluble. So here on adding zinc to CuSO4 solution zinc displaces copper from copper sulphate forms zinc sulphate solution. It also works with a wider range of resist materials than pure acids do. Anode dissolves in the electrolyte 4.
Combine 150 g CuSO45H2O 600 g sodium potassium tartrate and 500 ml distilled water in a beaker and stir add while stirring 300 ml of 10 NaOH wv transfer to a 1 liter volumetric flask and bring to 1 liter with distilled water.
While halides are generally soluble those of Ag are not. It is used to lower the pH of solutions. Copper in the soil may be from industry motor vehicle and architectural materials. B AgNO3 is soluble. As per the balanced equation molecules of ozone required for every one molecule of PbS isare a 4 b 3 c 2 d 1. Some heavy metals are VERY TOXIC POISONS especially if their salts are very soluble in water eg lead chromium mercury bismuth osmium and arsenic.
 Source: researchgate.net
Source: researchgate.net
Is Salt a Compound. In general salts of the alkali metals are soluble. Copper in the soil may be from industry motor vehicle and architectural materials. 2 NaBraq PbNO32aq– 2 NaNO3 PbBr2 yes NaNO3 is insoluble more information is required No both products are soluble yes both products are insoluble. Certain non-acids that form acid in water such as ferric chloride FeCl3 or copper sulfate CuSO4 can also be used as etching.
 Source: youtube.com
Source: youtube.com
Ii They have high melting points and boiling points. The observation when ammonium chloride reacts with potassium hydroxide. Hydrogen bonds are also formed between protein alpha and beta structures and water. Some heavy metals are VERY TOXIC POISONS especially if their salts are very soluble in water eg lead chromium mercury bismuth osmium and arsenic. The secondary structure of proteins depends largely on the interaction of peptide bonds with water through hydrogen bonds.
 Source: youtube.com
Source: youtube.com
A reddish brown gas 2. A tolerance of 1 ppm is established in potable water for residues of copper resulting from the use of the algicides or herbicides basic copper carbonate malachite copper sulfate copper monoethanolamine and copper triethanolamine to control aquatic plants in reservoirs lakes ponds irrigation ditches and other potential sources of potable water. The secondary structure of proteins depends largely on the interaction of peptide bonds with water through hydrogen bonds. Is Salt a Compound. The first reaction is much more rapid than the second.
 Source: youtube.com
Source: youtube.com
That has nothing to do with the redox reaction with copper sulfate because that reaction would involve evolution of Cu0 ie. This is indicated by colour change from blue to colourless. Add 5 mL distilled water to the sodium chloride. As per the balanced equation molecules of ozone required for every one molecule of PbS isare a 4 b 3 c 2 d 1. The secondary structure of proteins depends largely on the interaction of peptide bonds with water through hydrogen bonds.
 Source: youtube.com
Source: youtube.com
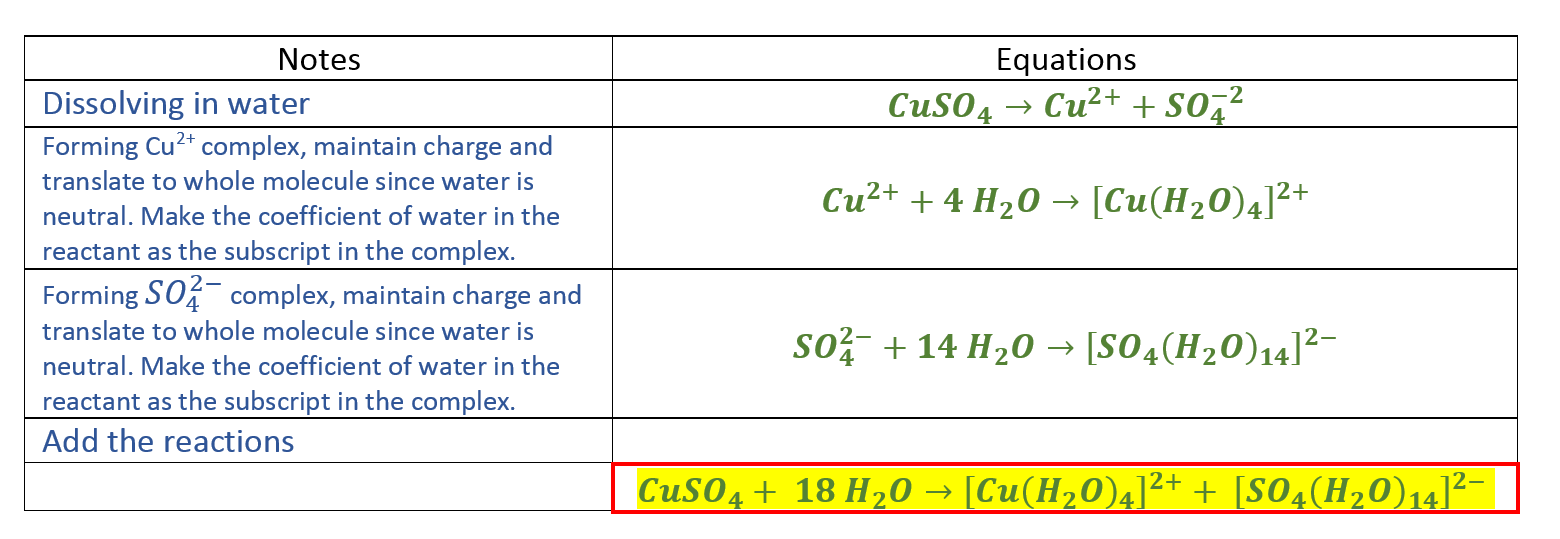
Add 5 mL distilled water to the sodium chloride. Ions produced when the compounds dissolve in water. As per the balanced equation molecules of ozone required for every one molecule of PbS isare a 4 b 3 c 2 d 1. IMMEDIATELY call a hospital or poison control center and locate activated charcoal egg whites or milk in case the medical advisor recommends administering one of them. The molecular equation contains which one of the following terms when balanced in standard form.

A displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. Copper in the soil may be from industry motor vehicle and architectural materials. The observation when ammonium chloride reacts with potassium hydroxide. Cu2S Fe2SO43 CuSO4 2FeSO4 CuS and. At the tertiary structure water.
 Source: tes.com
Source: tes.com
Dispose of this solution in the sink and rinse the beaker. C K2CO3 KI and KMnO4 are soluble. Cu2S Fe2SO43 CuSO4 2FeSO4 CuS and. 1 b 2. BaCl2 CuSO4 -.
 Source: youtube.com
Source: youtube.com
A typical example of a single displacement reaction where one metal displaces another is the reaction between iron and copper sulfate given by the reaction Fe CuSO 4 FeSO 4 Cu. In general salts of the alkali metals are soluble. Which of the following ionic compounds is soluble in water. BaSO4 CuCl2 Barium chloride reacts with copper sulphate to give barium sulphate and copper chloride. It is used in fertilizers and in processed food.
Source: quora.com
All the answers here sound perfectly reasonable until you look at the video and see rapid evolution of bubbles in the solution. 1 c 4. Test and record your results. Nitrate salts are soluble. A tolerance of 1 ppm is established in potable water for residues of copper resulting from the use of the algicides or herbicides basic copper carbonate malachite copper sulfate copper monoethanolamine and copper triethanolamine to control aquatic plants in reservoirs lakes ponds irrigation ditches and other potential sources of potable water.
 Source: clutchprep.com
Source: clutchprep.com
The molecular equation contains which one of the following terms when balanced in standard form. Answer 1 of 5. Its more commonly used to etch copper but it also works well to etch stainless steel. It is a good water absorbing agent. They are crystalline solids.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is cuso4 soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.