Is copper chloride soluble
Home » chemistry » Is copper chloride solubleIs copper chloride soluble
Is Copper Chloride Soluble. Copper oxide particles are practicably non-soluble at aqueous solutions with a pH 55 and above. The cyanide acts by reacting with copper in solution first reducing Cu 2 to Cu then forming soluble cupro-cyanide complexes CuCN x 1x eg CuCN 2 which are stable and prevent the uptake of copper by Eq. CopperII hydroxide is used to kill mold in paints. If the unknown is soluble do NOT perform solubility tests below Neutral acidic or basic.
 Copper Ii Chloride Wikipedia From en.wikipedia.org
Copper Ii Chloride Wikipedia From en.wikipedia.org
Chickpeas provide growing pets with protein fiber vitamin K copper zinc potassium and manganese. They are each made of a ca. While copper and nickel are mutually soluble with each other as shown in Figure 1 a number of other elements are not soluble in the Cu-Ni alloy and if present may cause cracking in the heat affected zone HAZ or weld metal. The survival of irradiated or unirradiated cells was not affected by the compound. It releases chlorine and turns into copperI chloride when heated very hot. However accidental or intentional copper overdose.
A typical example of a single displacement reaction where one metal displaces another is the reaction between iron and copper sulfate given by the reaction Fe CuSO 4 FeSO 4 Cu.
Answer 1 of 5. Answer 1 of 5. Most acids most. While copper and nickel are mutually soluble with each other as shown in Figure 1 a number of other elements are not soluble in the Cu-Ni alloy and if present may cause cracking in the heat affected zone HAZ or weld metal. Copper is an essential trace element that is included in some over-the-counter multivitamin and mineral supplements even though copper deficiency is quite rare and supplementation is rarely needed. The amounts of copper found in typical supplements has not been associated with serum enzyme elevations or with clinically apparent liver injury.
 Source: youtube.com
Source: youtube.com
Uses of Copper Sulfate. If this is the first. HCl Basic Most amines except III amines with only aromatic groups Soluble in dil. Drink the nutritional shake as a supplement snack or meal. It is an ionic compound of chlorine and calcium.
Source: en.wikipedia.org
CopperI chloride commonly called cuprous chloride is the lower chloride of copper with the formula CuClThe substance is a white solid sparingly soluble in water but very soluble in concentrated hydrochloric acidImpure samples appear green due to the presence of copperII chloride CuCl 2. Dried egg product is a highly digestible nutrient-rich ingredient that provides pets with protein and helps boost immunity. When an aqueous solution of sodium hydroxide NaOH is added to an aqueous solution of chromiumIII nitrate CrNO33 a precipitate of chromiumIII. HCl Basic Most amines except III amines with only aromatic groups Soluble in dil. Glucerna is the 1 doctor-recommended brand for people with diabetes.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Copper oxide particles are practicably non-soluble at aqueous solutions with a pH 55 and above. Reaction with chlorobenzene to give ferrous chloride. Copper oxide particles are practicably non-soluble at aqueous solutions with a pH 55 and above. Textile products impregnated with copper oxide particles continue to be efficacious even after 50 repeated home or industrial washings 56 86. Store unopened at room temperature.
 Source: en.wikipedia.org
Source: en.wikipedia.org
When an aqueous solution of sodium hydroxide NaOH is added to an aqueous solution of chromiumIII nitrate CrNO33 a precipitate of chromiumIII. If a precipitate forms the resulting precipitate is suspended in the mixture. Dried egg product is a highly digestible nutrient-rich ingredient that provides pets with protein and helps boost immunity. For example copper does not react with dilute acids so this metal cannot be used. In this case both of the reactants are salts.
 Source: sciencemadness.org
Source: sciencemadness.org
Questions to test your understanding. Shake well prior to opening. Copper sulfate is highly soluble in water with solubility values of 1055 molal and 1502 molal ate 10 o C and 30 o C respectively. Most acids most. Other mineral salts such as magnesium chloride are not absorbed as well but bioavailability still remains high.
 Source: en.wikipedia.org
Source: en.wikipedia.org
First you get copperI chloride formed. CopperII chloride is light brown when anhydrousIt is green when hydratedIt is a weak oxidizing agentIt reacts with aluminium foil to make hydrogen copperI oxide and aluminium chlorideThis is used in school demonstrations. Glucerna is the 1 doctor-recommended brand for people with diabetes. It is highly soluble in water and hence is hygroscopic in nature. It is essential that the surfaces be clean.
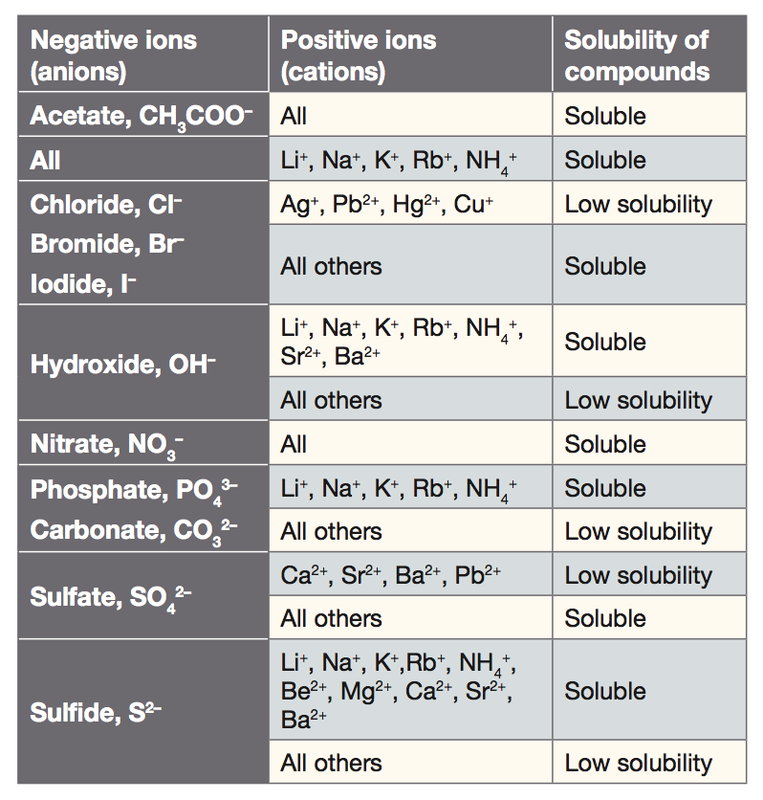 Source: anglesandacid.com
Source: anglesandacid.com
Uses of Copper Sulfate. It can be used as a catalyst. First you get copperI chloride formed. Ferric chloride shows following chemical reactions Reaction with iron III oxide. The comutagenic effects.
 Source: youtube.com
Source: youtube.com
If this is the first. It is an ionic compound of chlorine and calcium. FeCl 3 CuCl FeCl 2 CuCl 2. But in the presence of excess chloride ions from the HCl this reacts to give a stable soluble copperI complex. A typical example of a single displacement reaction where one metal displaces another is the reaction between iron and copper sulfate given by the reaction Fe CuSO 4 FeSO 4 Cu.
 Source: researchgate.net
Source: researchgate.net
Copper is an essential trace element that is included in some over-the-counter multivitamin and mineral supplements even though copper deficiency is quite rare and supplementation is rarely needed. Questions to test your understanding. While copper and nickel are mutually soluble with each other as shown in Figure 1 a number of other elements are not soluble in the Cu-Ni alloy and if present may cause cracking in the heat affected zone HAZ or weld metal. Lead sulfur and phosphorus are particularly detrimental and may cause intergranular hot cracking in highly restraint joints. FeCl 3 CuCl FeCl 2 CuCl 2.
 Source: youtube.com
Source: youtube.com
Copper chloride acted as comutagen in E coli WP2 which has wild type DNA repair capability but was much less comutagenic in the repair deficient strain WP2S uvra. It is an ionic compound of chlorine and calcium. I will give the lowest level of explanation and then follow up with a high level explanation. The major soluble salts copperII sulfate copperII chloride are generally more toxic than the less soluble salts. The cyanide acts by reacting with copper in solution first reducing Cu 2 to Cu then forming soluble cupro-cyanide complexes CuCN x 1x eg CuCN 2 which are stable and prevent the uptake of copper by Eq.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is copper chloride soluble by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.