Is chromium nitrate soluble
Home » chemistry » Is chromium nitrate solubleIs chromium nitrate soluble
Is Chromium Nitrate Soluble. The most widely applied nitrogen fertilizers are probably NaNO 3 sodium nitrate and NH 4 NO 3 ammonium nitrate. Alkaline Hypobromite Oxidation 8024. This page looks at some aspects of chromium chemistry required for UK A level and its equivalents. COD LR Dichromate 8000.
Chromium Iii Nitrate Crn3o9 Chemspider From chemspider.com
Yes no If a reaction does occur write the net ionic equation. The acute and subacute toxicities of several CrIII and CrVI compounds chromium3 chloride chromium3 nitrate chromium3 sulfate chromium trioxide potassium dichromate were determined in NZC and CxO mice injected ipThe distal median lethal doses 10 days after treatment averaged 179 or - 18 X 10-6 g chromiumg body wt regardless of the oxidation state of the Cr. Use the solubility. EPA sets legal limits on over 90 contaminants in drinking water. Beside above does a reaction occur when aqueous solutions of Chromium II chloride and lead II nitrate are combined. Nickel Soluble salts 100 4 100 Specific Nitrate as N 10000 100 10000 Specific Nitrate and Nitrite nn 10000 10 10000 Specific Nitrite 1000 10 1000 Specific Nitrobenzene 4 6 6 Specific NNitrosodimethylamine 00007 08 08 Specific NNitrosodinpropylamine Din propylnitrosamine 0.
Yes no If a reaction does occur write the net ionic equation.
003 - 100 mgL TNT854 Chromium Total. 15 Diphenylcarbohydrazide 10218 10219. It might than play an important role in the carbon cycle. 003 - 100 mgL TNT854 Chromium Total. Alkaline Hypobromite Oxidation 8024. Exposure to chromium causes several toxic effects namely dermatitis allergies cancer mutations and teratogenic effects which have been attributed to hexavalent chromium ions Cr VI.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Method III Ion-Selective Electrode Method Method B. The residue ends up in groundwater and surface water through soils because nitrates are water soluble. COD LR Dichromate 8000. Hexavalent chromium chromiumVI CrVI chromium 6 is chromium in any chemical compound that contains the element in the 6 oxidation state thus hexavalentVirtually all chromium ore is processed via hexavalent chromium specifically the salt sodium dichromateHexavalent chromium is key to all materials made from chromium. 1g28 ml water 25 C.
 Source: youtube.com
Source: youtube.com
After fertilization crops take up a relatively small part of added nitrogen compounds namely 25-30. ChromiumIII hydroxide Cr. Method III Ion-Selective Electrode Method Method B. Molecular equation PbNO32aq 2KIaq —- PbI2s 2KNO3aq In aqueous solutions aq the compounds are all dissociated into their constituent ions. Chromium Hexavalent and Total TNTplus 1.
 Source: youtube.com
Source: youtube.com
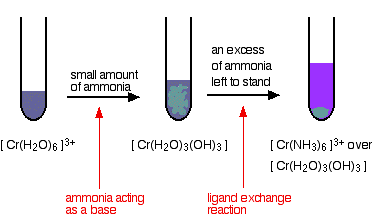
This is our newest publication and has been created to support the school technician profession in Scotland. Reactions of chromiumIII ions in solution summarised from elsewhere on the site. Even so you rarely ask someone to pass the sodium chloride at the dinner table. The acute and subacute toxicities of several CrIII and CrVI compounds chromium3 chloride chromium3 nitrate chromium3 sulfate chromium trioxide potassium dichromate were determined in NZC and CxO mice injected ipThe distal median lethal doses 10 days after treatment averaged 179 or - 18 X 10-6 g chromiumg body wt regardless of the oxidation state of the Cr. 003 - 100 mgL TNT854 Chromium Total.
 Source: en.wikipedia.org
Source: en.wikipedia.org
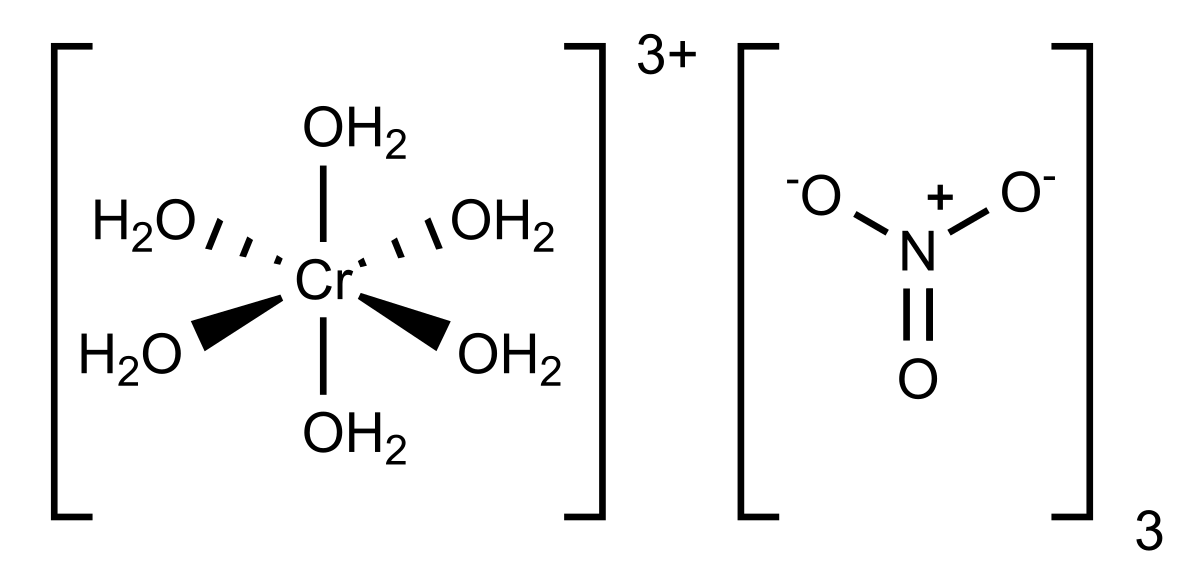
Use the solubility. The most widely applied nitrogen fertilizers are probably NaNO 3 sodium nitrate and NH 4 NO 3 ammonium nitrate. It is found in sewage and wastes from human andor farm animals and generally gets into drinking water from these activities. Exposure to chromium causes several toxic effects namely dermatitis allergies cancer mutations and teratogenic effects which have been attributed to hexavalent chromium ions Cr VI. Chromium trinitrate is an inorganic nitrate salt consisting of chromium and nitrate in which the ratio of chromium in the 3 oxidation state to nitrate is 13 It is.
Source: chemspider.com
The residue ends up in groundwater and surface water through soils because nitrates are water soluble. After fertilization crops take up a relatively small part of added nitrogen compounds namely 25-30. Easily soluble in hot water. A large number of chromiumIII compounds are known such as chromiumIII nitrate chromiumIII acetate and chromiumIII oxide. This kind of reaction is also observed with solutions of chrome alum and other water-soluble chromiumIII salts.
 Source: chemkits.eu
Source: chemkits.eu
And the structure of chromate oxyanions is like that of sulfate and phosphate ions which. 3 - 150 mgL. The residue ends up in groundwater and surface water through soils because nitrates are water soluble. Nitrate MCL 10 mgL as Nitrogen EPA US 2006 contamination comes through fertilizers. Use the solubility.
 Source: chemguide.co.uk
Source: chemguide.co.uk
This deviation was justified by the 2003 hierarchy memo PDF 4 pp 25 K that acknowledges and recognizes that EPA should use the best science available on which to base risk assessments In. This is a deviation from the typical RSL toxicity hierarchy. Even so you rarely ask someone to pass the sodium chloride at the dinner table. This kind of reaction is also observed with solutions of chrome alum and other water-soluble chromiumIII salts. Exposure to chromium causes several toxic effects namely dermatitis allergies cancer mutations and teratogenic effects which have been attributed to hexavalent chromium ions Cr VI.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Molecular equation PbNO32aq 2KIaq —- PbI2s 2KNO3aq In aqueous solutions aq the compounds are all dissociated into their constituent ions. Chromium Hexavalent and Total TNTplus 1. Method II Ion-Selective Electrode Method Method A. 1g28 ml water 25 C. Soluble Salts or Total Dissolved Solids.
 Source: chegg.com
Source: chegg.com
Chromium trinitrate is an inorganic nitrate salt consisting of chromium and nitrate in which the ratio of chromium in the 3 oxidation state to nitrate is 13 It is. The iron cycle means reduction of tertiary iron by organic ligands a process that is photo. Method III Ion-Selective Electrode Method Method B. This page looks at some aspects of chromium chemistry required for UK A level and its equivalents. Method II Ion-Selective Electrode Method Method A.
 Source: brainly.com
Source: brainly.com
The iron cycle means reduction of tertiary iron by organic ligands a process that is photo. This is a deviation from the typical RSL toxicity hierarchy. Method III Ion-Selective Electrode Method Method B. PH Hardness Aluminum Calcium Chromium Copper Iron Magnesium Manganese and Zinc Annually after initial comprehensive water chemistry Additional Testing Recommendations Verification of Potential Contamination Lead and. Loss on Ignition.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is chromium nitrate soluble by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.