Is barium oxide soluble in water
Home » chemistry » Is barium oxide soluble in waterIs barium oxide soluble in water
Is Barium Oxide Soluble In Water. Nitrate salts are soluble. About the Societies. Barium is a soft silvery-white metal with a slight golden shade when ultrapure. AgI and Ag3PO4 are not soluble.
 Is Barium Oxide Soluble In Water Brainly In From brainly.in
Is Barium Oxide Soluble In Water Brainly In From brainly.in
Phosphate salts are generally insoluble. About the Societies. In sulfate form however barium is not water soluble. Barium is used in many industrial processes as well as in diagnostic testing fireworks and pesticides. Because barium is difficult to purify many of its properties have not been accurately determined. Barium is toxic as are its water- or acid-soluble compounds.
Phosphate salts are generally insoluble.
Barium also carries another similarity to lead. Excessive quantities of barium oxide may lead to death. B AgNO3 is soluble. Barium also carries another similarity to lead. C K2CO3 KI and KMnO4 are soluble. 212 contains zinc oxide rosin and zinc acetate in the powder.
 Source: chegg.com
Source: chegg.com
Nitrate salts are soluble. B AgNO3 is soluble. 2 The silvery-white color of barium metal rapidly vanishes upon oxidation in air yielding a dark gray layer containing the oxideBarium has a medium specific weight and high electrical conductivity. The rosin increases fracture resistance and the zinc acetate is effective in accelerating the reaction rate. Barium is toxic as are its water- or acid-soluble compounds.

Another complex oxide barium titanate BaTiO 3 is used in capacitors as a piezoelectric material and in nonlinear optical applications. Barium also carries another similarity to lead. Barium occurs only in combination with other elements. The liquid is a preparation of eugenol which reacts with the powder to form an amorphous chelate of zinc eugenolate. Because barium is difficult to purify many of its properties have not been accurately determined.
Source: quora.com
This is called radiocontrast. While halides are generally soluble those of Ag are not. Barium metal can be prepared by electrolysis of molten barium chloride or by heating barium oxide with aluminium powder. Barium also carries another similarity to lead. The liquid is a preparation of eugenol which reacts with the powder to form an amorphous chelate of zinc eugenolate.
 Source: researchgate.net
Source: researchgate.net
Barium is a soft silvery-white metal with a slight golden shade when ultrapure. Nitrate salts are soluble. AgI and Ag3PO4 are not soluble. Barium is a naturally occurring alkaline metalloid element with atomic symbol Ba atomic number 56 and atomic weight 137 that is only found in combination with other elements typically barite barium sulfate and witherite barium carbonate or chemicals. The liquid is a preparation of eugenol which reacts with the powder to form an amorphous chelate of zinc eugenolate.
 Source: brainly.in
Source: brainly.in
This is called radiocontrast. Another complex oxide barium titanate BaTiO 3 is used in capacitors as a piezoelectric material and in nonlinear optical applications. This is called radiocontrast. All soluble barium compounds are toxic to mammals. Barium is a naturally occurring alkaline metalloid element with atomic symbol Ba atomic number 56 and atomic weight 137 that is only found in combination with other elements typically barite barium sulfate and witherite barium carbonate or chemicals.
 Source: scielo.org.za
Source: scielo.org.za
Phosphate salts are generally insoluble. Barium also carries another similarity to lead. The lack of this solubility causes it to simply. In sulfate form however barium is not water soluble. Barium metal can be prepared by electrolysis of molten barium chloride or by heating barium oxide with aluminium powder.
 Source: en.wikipedia.org
Source: en.wikipedia.org
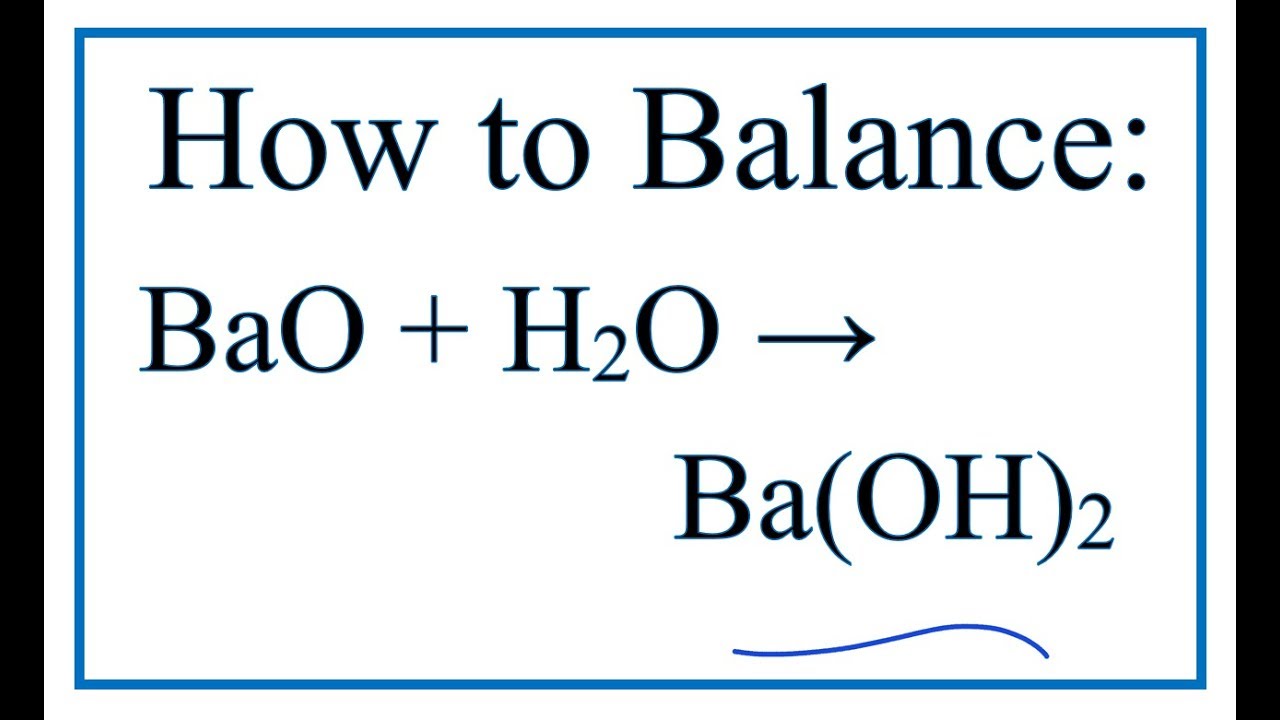
Barium hydroxide may be prepared a By dissolving barium oxide in water with subsequent crystallization b By precipitation from an aqueous solution of the sulfide by caustic soda c By heating barium sulfide in earthenware retorts into which a current of moist carbonic acid is passed after which superheated steam is passed over the resulting heated carbonate. While halides are generally soluble those of Ag are not. Barium chloride BaCl 2 2H 2 O consisting of colourless crystals that are soluble in water is used in heat-treating baths and in laboratories as a chemical reagent to precipitate soluble sulfates. The impetus of the membership remains research-based academic surgery and to promote the shared vision of research and academic pursuits through the exchange of ideas between senior surgical residents junior faculty and established academic surgical professors. Zinc oxideeugenol cement Fig.
 Source: youtube.com
Source: youtube.com
212 contains zinc oxide rosin and zinc acetate in the powder. Water soluble while oxides and carbonates are not. Barium metal can be prepared by electrolysis of molten barium chloride or by heating barium oxide with aluminium powder. Nitrate salts are soluble. The barium coating will be illuminated on the X-ray allowing for the diagnoses of certain abnormalities in the stomach esophagus intestines or colon.
 Source: youtube.com
Source: youtube.com
Barium is a soft silvery-white metal with a slight golden shade when ultrapure. Nitrate salts are soluble. Barium oxide BaO baria is a white hygroscopic non-flammable compoundIt has a cubic structure and is used in cathode ray tubes crown glass and catalystsIt is harmful to human skin and if swallowed in large quantity causes irritation. About the Societies. Help text not available for this section currently History.
 Source: youtube.com
Source: youtube.com
2 The silvery-white color of barium metal rapidly vanishes upon oxidation in air yielding a dark gray layer containing the oxideBarium has a medium specific weight and high electrical conductivity. In general salts of the alkali metals are soluble. The impetus of the membership remains research-based academic surgery and to promote the shared vision of research and academic pursuits through the exchange of ideas between senior surgical residents junior faculty and established academic surgical professors. C K2CO3 KI and KMnO4 are soluble. The rosin increases fracture resistance and the zinc acetate is effective in accelerating the reaction rate.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is barium oxide soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.