Is barium chromate soluble
Home » chemistry » Is barium chromate solubleIs barium chromate soluble
Is Barium Chromate Soluble. Reaction with nitric acid. Most strontium is used as the carbonate in special glass for television. Acetic acid due to the formation of soluble barium acetate. Barium chromate BaCrO 4 117 1010 Barium fluoride BaF 2 184 107 Barium hydroxide octahydrate BaOH 2 8H 2 O 255 104 Barium iodate BaIO 3 2 401 109 Barium iodate monohydrate BaIO 3 2 H 2 O 167 109 Barium molybdate BaMoO 4 354 108 Barium nitrate BaNO 3 2 464 103 Barium selenate.
 Barium Chromate Powder Bacro4 Cas No 10294 40 3 From samaterials.com
Barium Chromate Powder Bacro4 Cas No 10294 40 3 From samaterials.com
With the exceptions of compounds of calcium Ca 2 strontium Sr 2 barium Ba 2 and the ions listed in rule 6 all sulfates are soluble. Most solutes become more soluble in a liquid as the temperature is increased. Exempt when mixed in food or animal feed. If available The stockroom will supply us with two sealed glass flasks containing nitrogen dioxide and dinitrogen tetroxide gases. The spectator ions in the following reaction isare. Magnesium sulfide decomposes in water.
Principal uses of strontium compounds are in pyrotechnics for the brilliant reds in fireworks and warning flares and in greases.
These two gases are at equilibrium. For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation. Exempt except when inhalable dust is present or can be generated through use. If youd like proof see how well instant coffee mixes in a cup of cold water compared to a cup of hot water. A little is used as a getter in vacuum tubes to remove the last traces of air. With the exceptions of compounds of calcium Ca 2 strontium Sr 2 barium Ba 2 and the ions listed in rule 6 all sulfates are soluble.
 Source: clutchprep.com
Source: clutchprep.com
For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation. Crude form of sodium carbonate. If it did not dissolve in water but appears to be. Barium acetate formed by dissolving barium carbonate in dil. But CrVI is much more toxic and forms compounds that are reasonably soluble in water.
 Source: toppr.com
Source: toppr.com
B Potassium permanganate test. A little is used as a getter in vacuum tubes to remove the last traces of air. Barium is a chemical element with the symbol Ba and atomic number 56. K sp M n m A m- n. Now add a 6M HCl solution drop by drop and observe.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Immediate steps should be taken to limit its spread to the environment. It is the fifth element in group 2 and is a soft silvery alkaline earth metalBecause of its high chemical reactivity barium is never found in nature as a free element. Which one of the following statements is. It is a known oxidizing agent and produces a. The primary hazard is the threat to the environment.
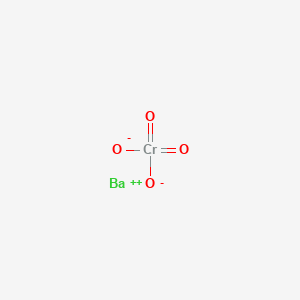
Most strontium is used as the carbonate in special glass for television. The colour of. 108 x 10-10. ChromateVI ions will give a yellow precipitate of barium chromateVI. 05 mol dm-3 barium nitrate solution 05 mol dm-3 sodium sulphate solution 05 mol dm-3 leadII nitrate solution 05 mol dm-3 potassium iodide solution 05 mol dm-3 potassium chromateVI solution and filter paper.
Source: sarthaks.com
In the test-tube the colour changes are. Most of the oxides of Groups 1 and 2 react with water to form hydroxides. Confirmation of Sulphite SO 3 2- a Barium chloride test. Strontium has uses similar to those of calcium and barium but it is rarely employed because of its higher cost. Acetic acid due to the formation of soluble barium acetate.
 Source: bengislife.com
Source: bengislife.com
Sulphites on reaction with barium chloride to form a white precipitate of barium sulphite. The spectator ions in the following reaction isare. Reaction with nitric acid. If the compound dissolved in water it should dissolve in nitric acid. The most common minerals of barium are baryte barium sulfate BaSO 4 and witherite barium carbonate BaCO 3The name barium originates from the.
 Source: samaterials.com
Source: samaterials.com
Immediate steps should be taken to limit its spread to the environment. HCl with the evolution of sulphur dioxide gas. Exposure to small amounts of CrVI can lead to damage of the respiratory gastrointestinal and immune systems as well as the kidneys liver blood and skin. Beakers filter funnel retort stand and clamp glass rod and 100 cm 3 measuring cylinder. Most solutes become more soluble in a liquid as the temperature is increased.
 Source: youtube.com
Source: youtube.com
The least-soluble compounds such as lead chromates or barium chromate are relatively less toxic because less hexavalent chromium is likely to be released into the body. Immediate steps should be taken to limit its spread to the environment. M m A n s mM n aq nA m-aq. BaIO 3 2 x H 2 O. 108 x 10-10.
 Source: en.wikipedia.org
Source: en.wikipedia.org
05 mol dm-3 barium nitrate solution 05 mol dm-3 sodium sulphate solution 05 mol dm-3 leadII nitrate solution 05 mol dm-3 potassium iodide solution 05 mol dm-3 potassium chromateVI solution and filter paper. Reaction with nitric acid. Atomic absorption spectroscopy has become one of the most frequently used tools in analytical chemistry. The precipitate colour is very similar to the background colour I use on Chemguide pages which makes the last diagram a bit difficult to see. Exempt when mixed in food or animal feed.
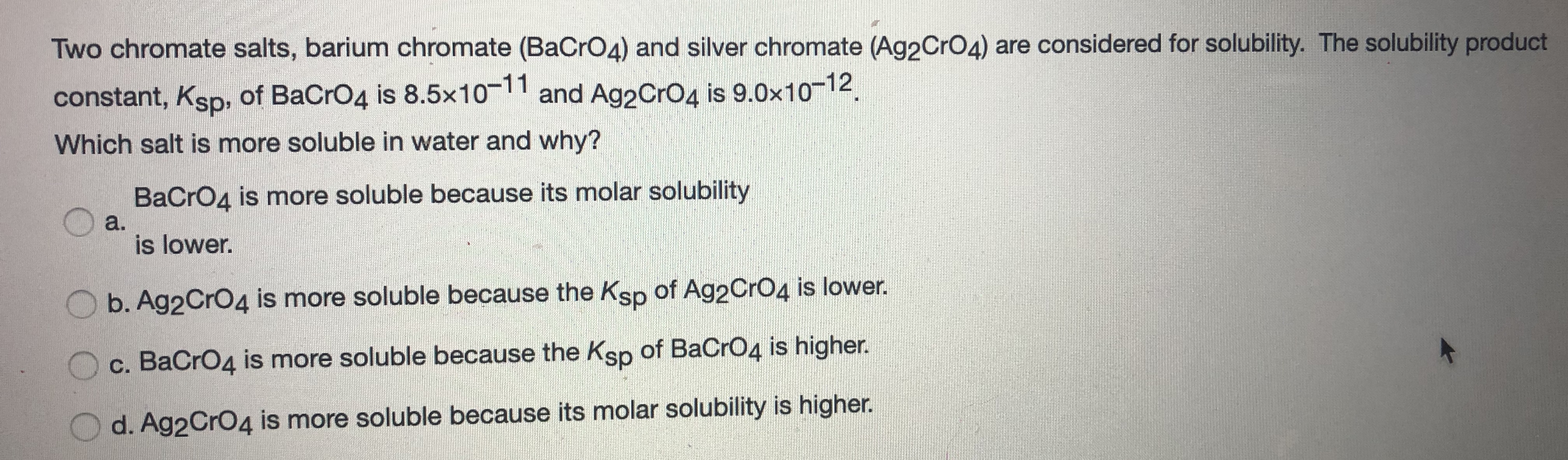 Source: bartleby.com
Source: bartleby.com
BaOH 2 x 8H 2 O. Toxicity varies with how soluble how easily they dissolve in water. The hydroxides of calcium strontium and barium are moderately soluble. Effect of temperature on equilibria. Acetic acid reacts with potassium chromate to form yellow precipitate of barium chromate.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is barium chromate soluble by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.