Is ammonium carbonate soluble in water
Home » chemistry » Is ammonium carbonate soluble in waterIs ammonium carbonate soluble in water
Is Ammonium Carbonate Soluble In Water. Salinity Laboratory Staff 1954 a saline soil has an EC of the saturated paste extract of more than 4 dSm a. Ammonium nitrate is a crystalline solid having a whitegrey colour. The ammonium fraction is taken up by roots or gradually converted to nitrate by soil microorganisms. The carbonate ion is the simplest oxocarbon anion consisting of one carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement.
 Is Nh4 2co3 Soluble Or Insoluble In Water Youtube From youtube.com
Is Nh4 2co3 Soluble Or Insoluble In Water Youtube From youtube.com
1105 g100 ml of water at 20 0 C. Ammonium nitrate is a crystalline solid having a whitegrey colour. Salinity Laboratory Staff 1954 a saline soil has an EC of the saturated paste extract of more than 4 dSm a. Alkaline earth Group IIA metals ions which are sufficiently soluble. The primary hazard is the threat to the environment. Solubility of NaCl is 36 g100 ml of water at 50 0 C.
Look the difference of two values.
When ions play this role in a reaction we call them spectator ions. Urea 46 N. It is also known as sal ammoniac the salt of ammonia and hydrogen chloride. Calcium carbonate precipitates as a solid leaving ammonium sulfate in the solution. Ammonium salts are very soluble and do not cause a RO scaling problem. The degree of ionization of ammonia to ammonium is dependent on pH temperature and the ionic strength of the solution.
 Source: chegg.com
Source: chegg.com
Other halides of. The carbonate ion CO3 A divalent complex anion. Silver chlorides solibility is in micro grams scale very small. Used to make other ammonium compounds in food processing and. 164 g100 ml of water at 15 0 C.
 Source: youtube.com
Source: youtube.com
In its pure form it is crystalline salt white. NH4Cl is an inorganic compound with the chemical name Ammonium Chloride. 164 g100 ml of water at 15 0 C. Ammonium cation is found in a variety of salts such as ammonium carbonate ammonium chloride and ammonium nitrateMost simple ammonium salts are very soluble in water. Immediate steps should be taken to limit spread to the environment.
Because of the calcium carbonate present it does not cause acidity when added to the soil. Ammonium Chloride is a systemic and urinary acidifying salt. Adding CuBr to H forms a weak acid HBr. The primary hazard is the threat to the environment. Water Water is a liquid.
 Source: youtube.com
Source: youtube.com
The ammonium fraction is taken up by roots or gradually converted to nitrate by soil microorganisms. Ammonium chloride helps maintain pH and exerts a. It is quite soluble in water. It has diuretic and expectorant effects. If a small amount of a dye such as Eriochrome black T is added to an aqueous solution containing calcium and magnesium ions at a pH of 10 01 the solution will become wine red.
 Source: bengislife.com
Source: bengislife.com
The common strong bases and their aqueous ions are. Why and how do you say AgCl is not soluble in water. You know only alkali metal carbonate compounds except lithium carbonate are soluble in water from metal carbonate compounds. In general long-chain APP starts to degrade at a temperature of. Ammonium bicarbonate appears as a white crystalline solid having the odor of ammonia.

The standard procedure for salinity testing is to measure EC of a solution extracted from a soil wetted to a saturation paste According to US. The ammonium ion NH4 A monovalent atomic anion. Ammonium cation is found in a variety of salts such as ammonium carbonate ammonium chloride and ammonium nitrateMost simple ammonium salts are very soluble in water. Water Water is a liquid. The solubility increases to 1024g100ml when the temperature is raised to 100o.
 Source: icandochemistry.com
Source: icandochemistry.com
Adding CuBr to H forms a weak acid HBr. Ammonium nitrate is a popular fertilizer since it provides half of the N in the nitrate form and half in the ammonium form. This is a short standard class experiment. Water is made of water molecules. Because pure water is a poor conductor of electricity increases in soluble salts result in proportional increases in the solution EC.
 Source: youtube.com
Source: youtube.com
The common strong bases and their aqueous ions are. N 100 crystalline form I is water soluble or water sensitive while APP with longer chains n 1000 crystalline form II displays a very low water solubility. It is used to make other ammonium compounds as a soldering flux as a fertilizer and for many other uses. 23 CO aq Lithium carbonate 2 Li-aq CO 3 2aq Na 34 PO aq Sodium phosphate 3 Na -aq PO 4 3aq NH 42 SO 4 aq Ammonium sulfate 2 NH 4 -aq SO 4 2aq 3. Thus it is highly soluble in.

Ammonium salts are very soluble and do not cause a RO scaling problem. The chloride ion Cl A monovalent molecular anion. This is a short standard class experiment. The exceptions are the alkali metals and the ammonium ion. It has diuretic and expectorant effects.
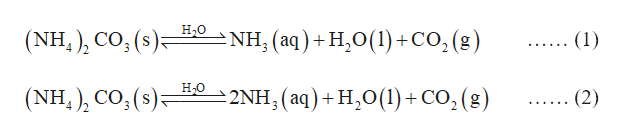 Source: bartleby.com
Source: bartleby.com
Immediate steps should be taken to limit its spread to the environment. Alkaline earth Group IIA metals ions which are sufficiently soluble. Other halides of. If EDTA is then added as a. You know only alkali metal carbonate compounds except lithium carbonate are soluble in water from metal carbonate compounds.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is ammonium carbonate soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.