Is 2 butanol soluble in water
Home » chemistry » Is 2 butanol soluble in waterIs 2 butanol soluble in water
Is 2 Butanol Soluble In Water. Examples of monosaccharides include glucose dextrose fructose levulose galactose xylose and ribose. All four compounds in the table are roughly the same size and all have moderate to excellent water solubility. If the total volume of waste to be disposed is greater than four liters per day approval by OCRS is required. Oligosaccharide An oligosaccharide is a saccharide polymer containing a small number typically 3-10 monosaccharides.
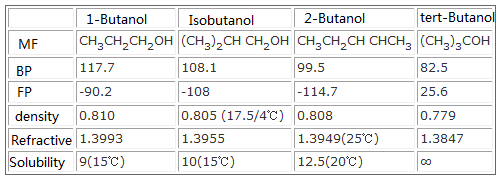
2-butanol 100 081 77 2-methyl-1-propanol 108 080 100 2-methyl-2-propanol 83 079 miscible 1-pentanol 138 082 27. TAA is mostly a positive allosteric modulator for GABA A receptors in the same way as ethanol. 2-Butanol or sec-butanol is an organic compound with formula C H 3 CHOHCH 2 CH 3. In the case of naphthalene the epoxide forms at the C-1 to C-2 bond rather than the C-2 to C-3 bond. Water-Soluble Phenols 1 Iron and the Human Body 1 iron spectrophotometrically 1 IronIII Chloride 1 it is possible to carryout a back titration. TAA is a colorless liquid with a burning flavor and an unpleasant odor similar to.
Examples of monosaccharides include glucose dextrose fructose levulose galactose xylose and ribose.
Dehydration of 2-butanol yields a 1-butene b 2-butene c 2-butyne d Both a and b Question. Tertiary Tertiary Secondary Secondary. This secondary alcohol is a flammable colorless liquid that is soluble in three parts water and completely miscible with organic solvents. Chemicals listed below must be in concentrations of approximately one percent or less to be suitable for sinksewer disposal. The carboxylic acids have pK a s near 45 and the conjugate acid of the amine has a pK a of 10. Disaccharides a sugar a carbohydrate composed of two monosaccharides.
 Source: bartleby.com
Source: bartleby.com
Since it is harder to break apart the bonds in an alcohol the boiling point will be higher. The hydroxyl group is referred to as a hydrophilic water-loving group because it forms hydrogen bonds with water and enhances the solubility of an alcohol in water. Ether and have relatively low melting points. Can be mixed with any of the solvents listed in the column at left Acetonitrile. Dehydration of 2-butanol yields a 1-butene b 2-butene c 2-butyne d Both a and b Question.
 Source: socratic.org
Source: socratic.org
The effects of TAA and ethanol are similar. The carboxylic acids have pK a s near 45 and the conjugate acid of the amine has a pK a of 10. Here the solution turns turbid or cloudy rapidly with the formation of two separate layers at room temperature eg. Soaking evacuated crystals in neat racemic 2-butanol at 40 C for two days led to the selective adsorption of R-2-butanol with one guest molecule bound within the cavity of each tetramer. Historically TAA has been used an anesthetic and more recently used as a recreational drug.
 Source: slideplayer.com
Source: slideplayer.com
Water-Soluble Phenols 1 Iron and the Human Body 1 iron spectrophotometrically 1 IronIII Chloride 1 it is possible to carryout a back titration. 2-butanol 100 081 77 2-methyl-1-propanol 108 080 100 2-methyl-2-propanol 83 079 miscible 1-pentanol 138 082 27. Hence this pathway does not lead to the accumulation of toxic by-products. Because alcohols form hydrogen bonds with water they tend to be relatively soluble in water. A 2-propanol B 15-pentanediol C 1-hexanol D 1-propanol E 2-butanol.

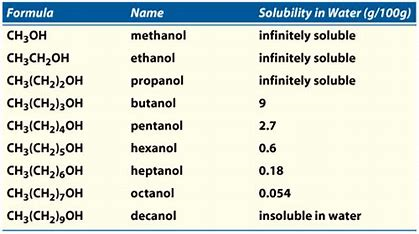
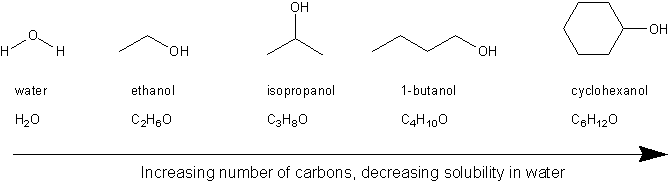
Short chain alcohols methanol ethanol isopropanol Insoluble Water solubility Soluble butanol pentanol. In Lucas test Zinc Chloride acts as catalyst. The hydroxyl group is referred to as a hydrophilic water-loving group because it forms hydrogen bonds with water and enhances the solubility of an alcohol in water. Can be mixed with any of the solvents listed in the column at left Acetonitrile. These MCQ for Class 12 Chemistry with Answers have been prepared based on the latest CBSE and NCERT syllabus and examination guidelines for Class 12 Chemistry.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A Predict the products of the following reactions b Name the starting materials and. Tertiary Tertiary Secondary Secondary. These MCQ for Class 12 Chemistry with Answers have been prepared based on the latest CBSE and NCERT syllabus and examination guidelines for Class 12 Chemistry. What was the percent by mass of water contained in the original container. Some monosaccharides have a sweet taste.
 Source: chegg.com
Source: chegg.com
Examples of monosaccharides include glucose dextrose fructose levulose galactose xylose and ribose. Alcohols have hydrogen bonding which is a much stronger bond than the London forces in normal hydrocarbons. Please use this book to increase your knowledge for the laboratory pratictioner. The first two are simple carboxylic acids and the third is an amino alcohol. 1 Jones Chromic Acid Oxidation Test for Aldehydes 1 Jones Oxidation 1 Kinetic Methods 1 kinetics 1 Lasker and Enkelwitz Test for Ketoses 1 Lindane 1 Lucas Test 1 Mean and Standard deviation -Arithmatic mean 1 methane 1.
 Source: researchgate.net
Source: researchgate.net
Alcohols of low molecular weight are a soluble in water b soluble in water on heating. These MCQ for Class 12 Chemistry with Answers have been prepared based on the latest CBSE and NCERT syllabus and examination guidelines for Class 12 Chemistry. Since it is harder to break apart the bonds in an alcohol the boiling point will be higher. Of 1390C but that of its isomer 2-methyl-2-butanol is 102oC. Historically TAA has been used an anesthetic and more recently used as a recreational drug.
 Source: chemicalbook.com
Source: chemicalbook.com
Record your results as very soluble for 6-10 drops soluble for 2-5 drops and insoluble 1 drop. These diols tend to be water-soluble and easily eliminated from the body. Since it is harder to break apart the bonds in an alcohol the boiling point will be higher. All four compounds in the table are roughly the same size and all have moderate to excellent water solubility. Organic compounds which are sulfur analogs of.
 Source: mendelset.com
Source: mendelset.com
These diols tend to be water-soluble and easily eliminated from the body. Urine was analyzed immediately 1 2 8 and 9 hr after drinking during 2 hr 375 mlkg of beverages containing orange juice 15 or 40 ethanol and 1 gl of 1-propanol 2-propanol 1-butanol 2-butanol isobutyl alcohol or a mixture of 1-propanol isobutyl alcohol. Can be mixed with any of the solvents listed in the column at left except. Repeat the same procedure using 2-propanol 1-butanol cyclohexanol phenol and unknown. 1920 Buprenorphine is commercially available as the brand.
 Source: researchgate.net
Source: researchgate.net
The major product obtained from dehydration of 2-hexanol is Question 9 options. All four compounds in the table are roughly the same size and all have moderate to excellent water solubility. Short chain alcohols methanol ethanol isopropanol Insoluble Water solubility Soluble butanol pentanol. O R H O H H H O H O H R H O R Alcohols also hydrogen-bond to each. 2-butanol is a clear organic secondary alcohol.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is 2 butanol soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.