Is 1 butanol soluble in water
Home » chemistry » Is 1 butanol soluble in waterIs 1 butanol soluble in water
Is 1 Butanol Soluble In Water. Organic compounds that are water soluble such as most of those listed in the above table generally have hydrogen bond acceptor and donor groups. These reactions can create fuel precursors. J Phys Chem Ref Data Monograph 1 p. Pressurized fluid extraction PFE soils sediments sludges solid waste Feb.
 Solved Why Was 1 Butanol More Soluble In Water Than In Chegg Com From chegg.com
Solved Why Was 1 Butanol More Soluble In Water Than In Chegg Com From chegg.com
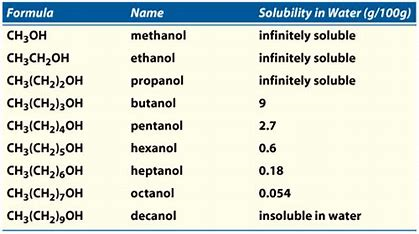
Is slightly soluble in water. CH 3 CH 2 4 OH. We find that diethyl ether is much less soluble in water. Ii The dissociation of water into ions is reversible. Alcohols are generally more soluble in water than alcohols of the same molecular mass. Isoamyl alcohol is a colorless liquid with the formula C 5 H 12 O specifically H 3 C 2 CHCH 2 CH 2 OH.
The core of a1-cbiss success is built with a strong emphasis on engineering design and technical experience supported by the industrys largest service network in the UK.
At room temperature ethers are pleasant-smelling colourless liquids. Water flush promptly Breathing. Is slightly soluble in water. This table shows that alcohols in red have higher boiling points and greater solubility in H 2 O than haloalkanes and alkanes with the same number of carbons. 1-butanol 118 081 91 2-butanol 100 081 77 2-methyl-1-propanol 108 080 100 2-methyl-2-propanol 83 079. Is it capable of forming hydrogen bonds with water.
 Source: socratic.org
Source: socratic.org
Select article Electric field tunable Li selectivity by eliminating coulomb blockage. Select article Electric field tunable Li selectivity by eliminating coulomb blockage. Low-molecular weight alcohols such as methanol and ethanol are miscible with water and solubility decreases as the number of carbons in the alcohol increases. Alcohols are generally more soluble in water than alcohols of the same molecular mass. Further analysis and comments regarding solubility and thermodynamic analysis of 16-Hexanediamine in mono-solvents and 1-butanol cyclohexane mixed solvents at different temperatures.
 Source: mendelset.com
Source: mendelset.com
The compounds that are more soluble in the. 1-butanol 118 081 91 2-butanol 100 081 77 2-methyl-1-propanol 108 080 100 2-methyl-2-propanol 83 079. The charges in one water molecule may be interacting with charges in other water molecules. The organic liquids that are also present in the developing solvent serve as the mobile phase. Environ Sci Technol 15.
 Source: researchgate.net
Source: researchgate.net
CH 3 CH 2 4 OH. The organic liquids that are also present in the developing solvent serve as the mobile phase. The reaction is catalyzed by a strong acid. B Actual microbial fuel cell showing the anode chamber left and cathode chamber right. In an alcohol one hydrogen atom of a water molecule is replaced by an alkyl group whereas in an ether both hydrogen atoms are replaced by alkyl or aryl groups.
 Source: bartleby.com
Source: bartleby.com
Low molecular-weight compounds are generally limited to those with fewer than five carbon atoms. 1-Butanol ACDIndex Name ACDIUPAC Name. PbOH 2 s Pb. Further analysis and comments regarding solubility and thermodynamic analysis of 16-Hexanediamine in mono-solvents and 1-butanol cyclohexane mixed solvents at different temperatures. Environ Sci Technol 15.
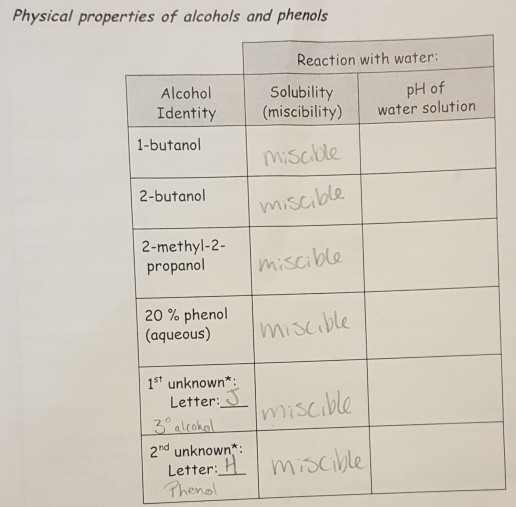
Water Solubility 1 2 and 3 amines can all form hydrogen bonds with water. 30-8 1981 3 Atkinson R. Medical attention immediately NIOSH EO1400000. Ii The dissociation of water into ions is reversible. Irritation eyes nose.
 Source: en.wikipedia.org
Source: en.wikipedia.org
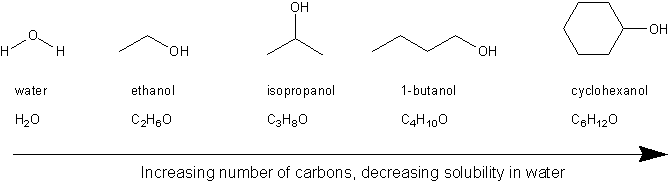
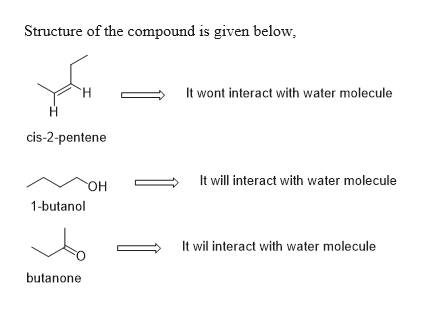
One difference between water and these other molecules is that water is polar. Is it capable of forming hydrogen bonds with water. There is a significant electronegativity difference between the oxygen and the hydrogen. Low-molecular weight alcohols such as methanol and ethanol are miscible with water and solubility decreases as the number of carbons in the alcohol increases. Irritation eyes nose.
 Source: chegg.com
Source: chegg.com
At the cathode microorganisms can convert the electrons to reduce oxygen to water under aerobic conditions or convert nitrate to nitrite or N 2 or convert CO 2 to acetate. Yes in fact it is the ether oxygen can. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. 30-8 1981 3 Atkinson R. Physical removal from air via wet deposition is possible since 2-methyl-1-butanol is relatively soluble in waterSRC.
 Source: researchgate.net
Source: researchgate.net
The core of a1-cbiss success is built with a strong emphasis on engineering design and technical experience supported by the industrys largest service network in the UK. J Phys Chem Ref Data Monograph 1 p. It also shows that the boiling point of alcohols increase with the number of carbon atoms. If skin irritation persists call a physician. Is slightly soluble in water.
 Source: hindawi.com
Source: hindawi.com
The effect of van der. Butan-1-ol is one of the fusel alcohols from the German for bad liquor which include alcohols that have more than two carbon atoms and have significant solubility in water. Water soluble organic compounds. 145 1989 Hazardous. The reaction is catalyzed by a strong acid.
 Source: researchgate.net
Source: researchgate.net
Hemisphere Pub Corp 1989 2 Eisenreich SJ et al. Most organic compounds are not soluble in water except for low molecular-weight amines and oxygen-containing compounds. It is also known as isopentyl alcohol isopentanol or in the IUPAC recommended nomenclature 3-methyl-butan-1-olAn obsolete name for it was isobutyl carbinol. This table shows that alcohols in red have higher boiling points and greater solubility in H 2 O than haloalkanes and alkanes with the same number of carbons. 145 1989 Hazardous.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is 1 butanol soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.