Cucl soluble or insoluble in water
Home » chemistry » Cucl soluble or insoluble in waterCucl soluble or insoluble in water
Cucl Soluble Or Insoluble In Water. Weve already seen that copperI iodide is produced as an off-white precipitate if you add potassium iodide solution to a solution containing copperII ions. Which chloride is water soluble. For most ionic compounds there is also a limit to the amount of compound can be dissolved in a sample of water. The equation Zn CuCl 2 Cu ZnCl 2 is an example of a _____ reaction.
 Is Cucl2 Soluble Or Insoluble In Water Youtube From youtube.com
Is Cucl2 Soluble Or Insoluble In Water Youtube From youtube.com
Such CuCl CuBr CuI and CuCN whose compounds are insoluble in water. 627 10 9. Copper sulfate is an inorganic salt that is highly soluble in water. For most ionic compounds there is also a limit to the amount of compound can be dissolved in a sample of water. Common Solubility Products by Decreasing Equilibrium Constants. Usually insoluble CO 3 2 PO 4 3 and OH Insoluble with some exceptions including with group 1 elements and NH 4 CaCO 3 Ca 3PO 4 2 and MnOH 2 are insoluble in water but NH 4 2CO 3 Li 3PO 4 and CsOH are.
Non-flammable NIOSH US health exposure limits.
The gas is insoluble in water. Preparation of Salts Preparation of salts in the laboratory The method used to prepare a salt depends on the solubility of the salt in water. Spermatozoa were immobilized after 20 minutes of exposure at the two highest concentrations. Synthesis takes advantage of the stability of solid CuCl which makes redox between Cu o and Cu 2. Substance A dissolves in water and is comprised of soluble ions. Copper sulfate is an inorganic salt that is highly soluble in water.
 Source: youtube.com
Source: youtube.com
Which chloride is water soluble. The solubility in an 0200 M solution of NaIO₃ of AgIO₃ is 15 10⁷ and the solubility of LaIO₃₃ is 94 10¹⁰. Which compound is more soluble. Which chloride is water soluble. You know that sodium chloride NaCl is soluble in water so the remaining product copper carbonate must be the one that is insoluble.
You cannot swap both. You would end up with the same substances you started with Either perspective should allow you to predict the proper products as long as you pair a cation with an anion and. The Ksp for AgIO₃ is 30 x 10⁸ and the Ksp for LaIO₃₃ is 75 x 10¹². Copper sulfate is an inorganic salt that is highly soluble in water. This can be observed in the compound cuprous chloride which is insoluble in water.
 Source: bengislife.com
Source: bengislife.com
This can be observed in the compound cuprous chloride which is insoluble in water. 7 10 29. The iodate ion has a number of insoluble compounds. A lead II chloride b calcium chloride c mercury I chloride d silver I chloride. SO 4 2 Soluble with some exceptions including with Ba2 and Pb2 FeSO 4 is water soluble but BaSO 4 is insoluble.
 Source: youtube.com
Source: youtube.com
PEL Permissible TWA 1 mgm 3 as Cu REL Recommended TWA 1 mgm 3 as Cu IDLH Immediate danger TWA 100 mgm 3 as Cu Related compounds Other anions. Spermatozoa were immobilized after 20 minutes of exposure at the two highest concentrations. Effect on Solubility. Another important moment is that the. However in concentrated chloride solutions the solubility rapidly increases 11.
 Source: slideplayer.com
Source: slideplayer.com
Hydrogen gas is lighter than air. 15 10 16. Solubility of CuCl in cold water is very low only around 60 mgl. 31 x 10-19 J nm x Q1Q2 r where Q is the ionic charge in atomic units and r is the distance between ions in nm Covalent Bonding When two atoms approach each other the result of the interaction depends on the relative strength of the following forces. Non-flammable NIOSH US health exposure limits.
Source: quora.com
AgBr is an ionic solid that is NOT soluble in water. Non-flammable NIOSH US health exposure limits. A combination b single replacement c double replacement d decomposition. You know that sodium chloride NaCl is soluble in water so the remaining product copper carbonate must be the one that is insoluble. The copperI iodide is virtually insoluble in water and so the disproportionation reaction doesnt happen.
 Source: slideplayer.com
Source: slideplayer.com
Decomposes in HNO 3 H 2 SO 4. CopperI oxide CopperI. Decomposes in HNO 3 H 2 SO 4. A AgIO₃ B LaIO₃₃. 31 x 10-19 J nm x Q1Q2 r where Q is the ionic charge in atomic units and r is the distance between ions in nm Covalent Bonding When two atoms approach each other the result of the interaction depends on the relative strength of the following forces.
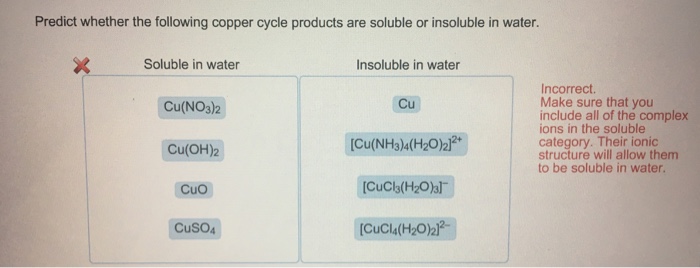
2 10 32. For example lead nitrate and sodium chloride are both soluble in water. A combination b single replacement c double replacement d decomposition. CBSE 2015 CBSE 2010 a. This compound can be dissolved in water by the addition of chloride ions leading to the formation of the CuCl 2 complex ion which is soluble in water.
 Source: bengislife.com
Source: bengislife.com
The Ksp for AgIO₃ is 30 x 10⁸ and the Ksp for LaIO₃₃ is 75 x 10¹². This fact is very important because the chloride technology of leaching sulphide minerals by CuCl 2 would be otherwise not feasible for use in practice. Synthesis takes advantage of the stability of solid CuCl which makes redox between Cu o and Cu 2. Substance A dissolves in water and is comprised of soluble ions. PbNO 3 2 s Pb2aq NO 3 aq NaCls Naaq Claq If these two solutions are combined then PbCl 2 s will precipitate form a solid from the combina-tion of two solutions.
 Source: youtube.com
Source: youtube.com
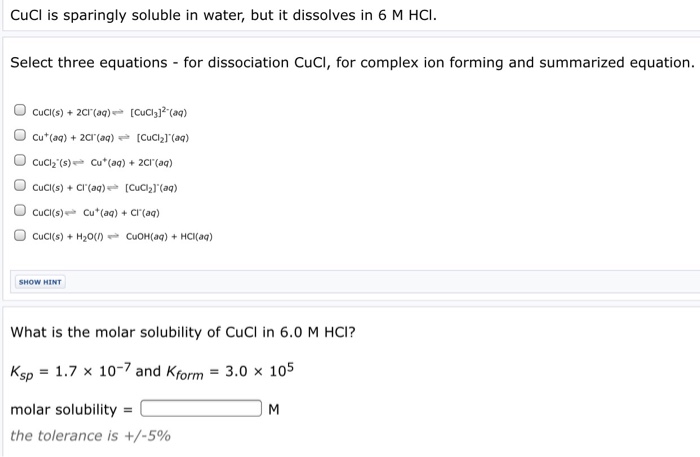
CuCl 2 aq 2 AgNO 3 aq Cu NO 3 2 aq 2 AgCls There are two equivalent ways of considering a double-replacement equation. Soluble in NH 4 OH. Insoluble substance cannot dissociate into ions in water. Writing Equations and Solubility Products Write the ionic equation for the dissolution and the solubility product expression for each of the following slightly soluble ionic. Which compound is more soluble.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title cucl soluble or insoluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.