Copper sulfate soluble or insoluble
Home » chemistry » Copper sulfate soluble or insolubleCopper sulfate soluble or insoluble
Copper Sulfate Soluble Or Insoluble. Barium Sulfate is composed of a barium cation and the sulfate anion. Did the copper change its charge in this reaction. Corneocyte a metabolically inactive. The form of the complex depends on.
 Temperature Dependence Of The Solubility Of Copper Sulfate In Water Download Scientific Diagram From researchgate.net
Temperature Dependence Of The Solubility Of Copper Sulfate In Water Download Scientific Diagram From researchgate.net
Are all precipitates insoluble in water. The amounts of copper found in typical supplements has not been associated with serum enzyme elevations or with clinically apparent liver injury. The blue color of the solution indicates that excess copperII sulfate remains after all of the sodium sulfide has reacted. To learn more about the Structure Properties Preparations Uses and FAQs of Sodium sulphate Visit BYJUS for detailed information. Death is preceded by gastric hemorrhage tachycardia hypotension hemolytic crisis convulsions and paralysis. However copper deficiency is likely to occur on organic soils mineral soils high in organic matter content 5 and on very sandy.
All alkaline earth metals forms insoluble carbonate.
On the other hand sodium is too. Ceruloplasmin a copper-containing protein facilitates the binding of ferric iron to transferrin via ferroxidase activity at the basolateral membrane Osaki et al 1966. Barium Sulfate is an alkaline divalent. Silver sulfate is slightly soluble. BaSO 4 is a sulfate salt of barium and is found as mineral barite. Students can then obtain blue copperII sulfate pentahydrate crystals.
 Source: researchgate.net
Source: researchgate.net
Formation of metallic copper In the fume hood add 20 g of powdered zinc metal stirring until any reaction is apparently complete. However basic samples should be avoided as hydroxide leaching into the electrolyte compartment will cause formation of insoluble copper hydroxide and copper oxide. Li 2 CO 3 is a white solid precipitate compound. In general when a solution of a soluble salt of the M m ion is mixed with a solution of a soluble salt of the X n ion the solid M p X q precipitates if the value of Q for the mixture of M m and X n is greater than K sp for M p X q. Solubility of carbonates have a variation because there are soluble and insoluble carbonates.

The copper ion is the component of copper sulfate with toxicological implications. Thus if we know the concentration of one of the ions of a slightly soluble ionic solid and the value for the solubility product of the solid then we. The insoluble reactant chosen depends upon the particular salt required. CopperII sulfate also known as copper sulphate are the inorganic compounds with the chemical formula Cu SO 4 H 2 O x where x can range from 0 to 5The pentahydrate x 5 is the most common form. Wollenberg et al 1990.
 Source: researchgate.net
Source: researchgate.net
The water-insoluble sulfates are also insoluble in dilute acids. The cyanide acts by reacting with copper in solution first reducing Cu 2 to Cu then forming soluble cupro-cyanide complexes CuCN x 1x eg CuCN 2 which are stable and prevent the uptake of copper by Eq. In this experiment students react an insoluble metal oxide with a dilute acid to form a soluble salt. Did the copper change its charge in this reaction. Similarly copperI chloride can be produced as a white precipitate reaction described below.
 Source: scielo.br
Source: scielo.br
The sulphur is attached to four oxygen atoms. Li 2 CO 3 is a white solid precipitate compound. Wollenberg et al 1990. Mercurous sulfate is slightly soluble in water 51 mg. On the other hand sodium is too.
 Source: researchgate.net
Source: researchgate.net
Anhydrous sodium sulfate is a white crystalline solid also known as the mineral thenardite. MgCrO4 is soluble in water. Anhydrous sodium sulfate is a white crystalline solid also known as the mineral thenardite. In general when a solution of a soluble salt of the M m ion is mixed with a solution of a soluble salt of the X n ion the solid M p X q precipitates if the value of Q for the mixture of M m and X n is greater than K sp for M p X q. Silver sulfate is slightly soluble.
 Source: docbrown.info
Source: docbrown.info
Precipitation Reactions The precipitate forms because the solid AgCl is insoluble in. This is a short standard class experiment. CopperII sulfate also known as copper sulphate are the inorganic compounds with the chemical formula Cu SO 4 H 2 O x where x can range from 0 to 5The pentahydrate x 5 is the most common form. However copper deficiency is likely to occur on organic soils mineral soils high in organic matter content 5 and on very sandy. The water-insoluble sulfates are also insoluble in dilute acids.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Silver sulfate is slightly soluble. Mercurous sulfate is slightly soluble in water 51 mg. Copper is an essential trace element that is included in some over-the-counter multivitamin and mineral supplements even though copper deficiency is quite rare and supplementation is rarely needed. The copperI iodide is virtually insoluble in water and so the disproportionation reaction doesnt happen. The cyanide acts by reacting with copper in solution first reducing Cu 2 to Cu then forming soluble cupro-cyanide complexes CuCN x 1x eg CuCN 2 which are stable and prevent the uptake of copper by Eq.
 Source: youtube.com
Source: youtube.com
Older names for this compound include blue vitriol bluestone vitriol of copper and Roman vitriol. All alkaline earth metals forms insoluble carbonate. The insoluble reactant chosen depends upon the particular salt required. Students can then obtain blue copperII sulfate pentahydrate crystals. The sulfides of all metals except barium.
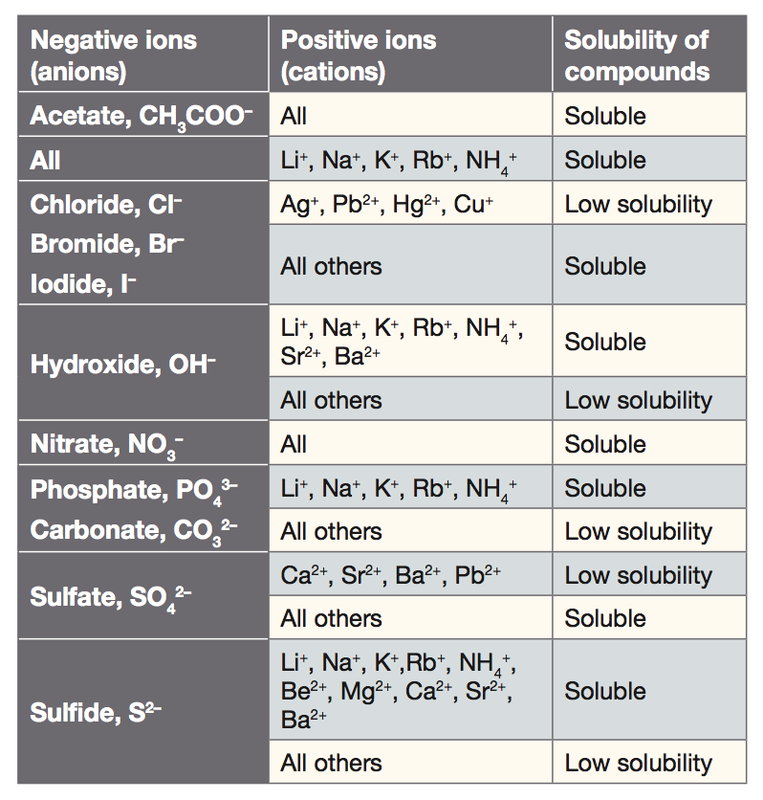 Source: anglesandacid.com
Source: anglesandacid.com
What color is copperII sulfate. Barium Sulfate is composed of a barium cation and the sulfate anion. But when we study deeply about solubility of metal carbonates most of the carbonates are insoluble in water. Thus if we know the concentration of one of the ions of a slightly soluble ionic solid and the value for the solubility product of the solid then we. Therefore the corrosion rate of copper in HCl particularly in moderate concentration is higher than H 2 SO 4 which is due to the formation of soluble compounds such as CuCl 2.
 Source: youtube.com
Source: youtube.com
Are all precipitates insoluble in water. Therefore the corrosion rate of copper in HCl particularly in moderate concentration is higher than H 2 SO 4 which is due to the formation of soluble compounds such as CuCl 2. 2O Soluble 20 Ferric sulfate FeSO 4 34H 2O Soluble 23 e. Barium sulfate or sulphate is the inorganic compound with the chemical formula Ba SO 4It is a white crystalline solid that is odorless and insoluble in waterIt occurs as the mineral barite which is the main commercial source of barium and materials prepared from it. Cornea the transparent covering of the front of the eye that transmits and focuses light into the eye.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title copper sulfate soluble or insoluble by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.