Copper carbonate soluble
Home » chemistry » Copper carbonate solubleCopper carbonate soluble
Copper Carbonate Soluble. PH Duodenum 549 553 545 006 071 Proximal jejunum 561 a 549 b 552 b 003 002 Ileum 643 a 696 b 695 b 008 00001. Older names for this compound include blue vitriol bluestone vitriol of copper and Roman vitriol. The result is a blue precipitate. In this section we will find out how we can control the dissolution of a slightly soluble ionic solid by the application of Le Châteliers principle.

For patients with hyperglycemiaabnormal glucose tolerance resulting from metabolic stress such as. It turns into copperII oxide when wet. Mineral form of basic copper carbonate. A brownish-red precipitate will be formed if ironIII Fe3 reacts with sodium hydroxide. Oxidised copper ore bodies may be treated via several processes with hydrometallurgical processes used to treat oxide ores dominated by copper carbonate minerals such as azurite and malachite and other soluble minerals such as silicates like chrysocolla or sulfates such as atacamite and so on. For example copper does not react with dilute acids so this metal cannot be used.
These copper oxide ores are usually.
Calcium carbonate 70 Salt 15 DL-Methionine. PH Duodenum 549 553 545 006 071 Proximal jejunum 561 a 549 b 552 b 003 002 Ileum 643 a 696 b 695 b 008 00001. 129 g100 ml of water at 25 0 C. It can also be made by reacting copperII hydroxide copperII oxide or copperII carbonate with hydrochloric acid and from pure copper and from 11 solution of hydrogen peroxide and hydrochloric acid where copper first get oxidized to CuO from H2O2 and then reacts with HCl to form CuCl2 reaction goes like this. 1105 g100 ml of water at 20 0 C. Alkaline earth metals forms.

Calculating the aqueous solubility of sparingly soluble compounds under various conditions. It turns into copperII oxide when wet. Thus if K sp Mm An is the equilibrium constant for the reaction M m A n s mMaq nA aq where M m A n is the slightly. Alkaline earth metals forms. For instance hydrometallurgical processes are used to treat copper oxide ores that are dominated by soluble minerals such as copper carbonate minerals.
 Source: en.wikipedia.org
Source: en.wikipedia.org
You simply get a precipitate of what you can think of as copperII carbonate. Mineral form of basic copper carbonate. Effect of amount and source of dietary copper on intestinal pH intestinal soluble intestinal mucosal bile and liver Cu concentration of weanling pigs. The result is a blue precipitate. 164 g100 ml of water at 15 0 C.
 Source: youtube.com
Source: youtube.com
Textile products impregnated with copper oxide particles continue to be efficacious even after 50 repeated home or industrial washings 56 86. 1 A great choice for patients who are trying to manage their blood glucose. Only one of the UK A level Exam Boards wants this. PH Duodenum 549 553 545 006 071 Proximal jejunum 561 a 549 b 552 b 003 002 Ileum 643 a 696 b 695 b 008 00001. It may be determined by direct measure- ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states.
 Source: youtube.com
Source: youtube.com
The result is a blue precipitate. It reacts with ammonia to make a very dark blue solution. It reacts with carbon dioxide to make copperII carbonate when in air. Therefore the corrosion rate of copper in HCl particularly in moderate concentration is higher than H 2 SO 4 which is due to the formation of soluble compounds such as CuCl 2. Only one of the UK A level Exam Boards wants this.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Calculating the aqueous solubility of sparingly soluble compounds under various conditions. Alkaline earth metals forms. You know only alkali metal carbonate compounds except lithium carbonate are soluble in water from metal carbonate compounds. The copper oxide particles serve as a reservoir of copper ions. LiF Li 2 CO 3 Li 2 C 2 O 4 are sparingly dissolve in water.
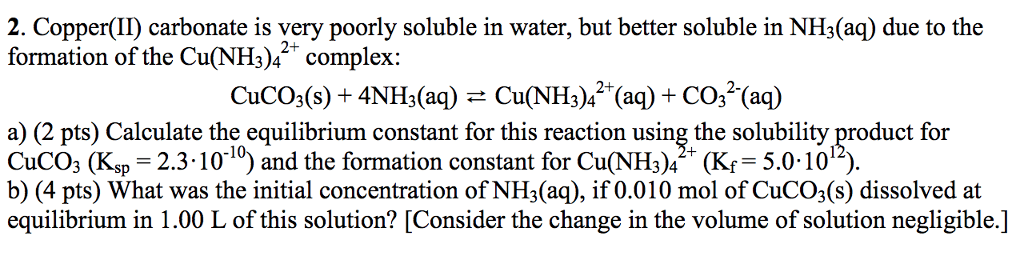 Source: oneclass.com
Source: oneclass.com
Calcium carbonate 70 Salt 15 DL-Methionine. It is made by reacting copper with chlorine. Methods for the analyses of solu ble sorbed and total concentrations of 34 elements are also included. CopperII sulfate also known as copper sulphate are the inorganic compounds with the chemical formula Cu SO 4 H 2 O x where x can range from 0 to 5The pentahydrate x 5 is the most common form. Textile products impregnated with copper oxide particles continue to be efficacious even after 50 repeated home or industrial washings 56 86.
 Source: en.wikipedia.org
Source: en.wikipedia.org
You know only alkali metal carbonate compounds except lithium carbonate are soluble in water from metal carbonate compounds. It turns into copperII oxide when wet. Therefore the corrosion rate of copper in HCl particularly in moderate concentration is higher than H 2 SO 4 which is due to the formation of soluble compounds such as CuCl 2. This fact explains the cave formation where the lime stone deposits have been in contact with acid waters. Copper hydroxide forms a blue precipitate.
 Source: slideplayer.com
Source: slideplayer.com
Additionally these chapters include useful background information on the chem istry of the elements. But in the presence of excess chloride ions from the HCl this reacts to give a stable soluble copperI complex. Therefore the corrosion rate of copper in HCl particularly in moderate concentration is higher than H 2 SO 4 which is due to the formation of soluble compounds such as CuCl 2. 164 g100 ml of water at 15 0 C. The presence of oxygen in the acidic solution causes increase in the corrosion attacks on copper.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Sodium hydrogen carbonate or sodium bicarbonate. It reacts with carbon dioxide to make copperII carbonate when in air. The pentahydrate CuSO 4 5H 2 O the most commonly encountered salt is bright blue. At the same time fluctuations in the levels of iron and zinc which are essential elements were determined in the tissuesLevels of copper iron and zinc in blood were. Therfore finding most solble metal compound is easier.
 Source: slideplayer.com
Source: slideplayer.com
Less solubilty of NaHCO 3 is very important factor in sodium carbonate manufacturing process by solvay process. The copper level increased progresively in mitochondria lysosomal fractions of the liver in proportion to the period of administration. Thus if K sp Mm An is the equilibrium constant for the reaction M m A n s mMaq nA aq where M m A n is the slightly. Although the calcium carbonate is very little soluble in water it is quite soluble if the water contains dissolved carbon dioxide for in these solutions it forms bicarbonate when dissolving. Therefore the corrosion rate of copper in HCl particularly in moderate concentration is higher than H 2 SO 4 which is due to the formation of soluble compounds such as CuCl 2.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title copper carbonate soluble by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.