Benzoic acid water soluble
Home » chemistry » Benzoic acid water solubleBenzoic acid water soluble
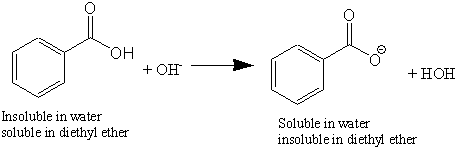
Benzoic Acid Water Soluble. The product was a white crystalline solid MP 114-122C and 121-127C respectively after recrystallization. 4-chloroaniline mp 68-71 or ethyl 4-aminobenzoate mp 90 Neutral options same choices for both Part 1 and Part 2. Once the solution is acidic place the flask in an ice-water bath for 5 minutes to complete the crystallization. For example benzoic acid is not soluble in water yet it is soluble in sodium hydroxide solution and in sodium hydrogen carbonate solution because these bases react with benzoic acid to form the water-soluble benzoate ion.
 Get Your Ba In Solubility Ochemonline From ochemonline.wordpress.com
Get Your Ba In Solubility Ochemonline From ochemonline.wordpress.com
Both of compounds are carboxylic acids. Benzoic acid is only sparingly soluble in cold acidic water. Sodium salts formed from the base extractions will be soluble in water while naphthalene will only be soluble in the original solvent diethyl ether. It is the simplest aromatic carboxylic acidThe name is derived from gum benzoin which was for a long time its only sourceBenzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. A stronger base sodium hydroxide is required to react with the less acidic 2-naphthol. Liquid Chromatographic Determination in Foods.
Determination According to SBR in Meat and Meat Products No.
When your sample is dry measure the mass and calculate your percent recovery. But benzoic acid is not soluble in water. This causes the solid to begin to form or precipitate. For example benzoic acid is not soluble in water yet it is soluble in sodium hydroxide solution and in sodium hydrogen carbonate solution because these bases react with benzoic acid to form the water-soluble benzoate ion. 4-Aminobenzoic acid also known as para-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the para position is an organic compound with the formula H 2 NC 6 H 4 CO 2 H. Benzoic Acid Sorbic Acid and p-Hydroxybenzoic Acid Esters.
Source:
Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. More specifically deprotonating benzoic acid forms a water-soluble carboxylate anion leaving the other organic by-products in the organic layer. A member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. It consists of a benzene ring substituted with amino. When your sample is dry measure the mass and calculate your percent recovery.
 Source: www2.chem.wisc.edu
Source: www2.chem.wisc.edu
Aspirin is only slightly soluble in water so if you add it to the reaction mixture the product would rather clump together than be in the water phase. Organic compounds soluble in organic solvents but not in water. They are more stable than benzoic acid more soluble in water and work best at pH levels between 2 and 44. It is slightly soluble in water. Benzoic acid mp 123 or 2-chlorobenzoic acid mp 141 Amine unknown options Part 2.
 Source: researchgate.net
Source: researchgate.net
This produces a water-soluble pleasant-smelling white powder that is used for flavorings and perfume. Any drug to be absorbed must be present in the form of an aqueous solution at the site of absorption. This can be done by pouring the entire contents of your flask through a fluted filter. Organic compounds soluble in organic solvents but not in water. Benzoic acid is an organic acid first used in foods almost 100 years ago.
 Source: ochemonline.wordpress.com
Source: ochemonline.wordpress.com
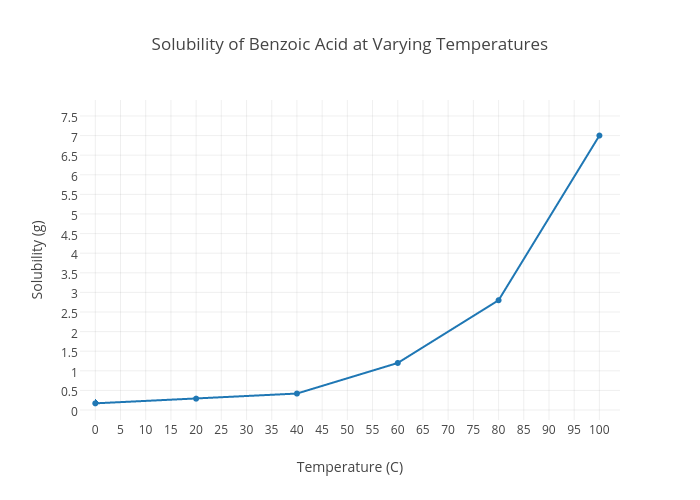
As we will learn when we study acid-base chemistry in a later chapter carboxylic acids such as benzoic acid are relatively weak acids and thus exist mostly in the acidic protonated form when added to pure water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. When heated to 100 degrees celsius the solubility of this compound in water increases to 5631 grams per. At a temperature of 0 degrees celsius the solubility of benzoic acid in water corresponds to 17 grams per litre. The three significant growth predictors for spoilage yeasts are the variables.
 Source: chart-studio.plotly.com
Source: chart-studio.plotly.com
When a basic solution is added to the funnel the acidic compound is deprotonated and becomes an ionic salt. Pahlavan 3 Example 1- The solubility of solid X in hot water 550 g100 ml at 100 o C is not very great and its solubility in cold water 053 g100ml at 0 o C is significant. Introducing the two sodium salts to hydrochloric acid will effectively replace the original proton benzoic acid and 2-naphthol lost. The three significant growth predictors for spoilage yeasts are the variables. Both of compounds are carboxylic acids.
 Source: degruyter.com
Source: degruyter.com
Salts of benzoic acid are used as food. Journal of Applied Spectroscopy reports on key applications of spectroscopy in physics chemistry material science medicine biology ecology and spectral instrument-industry. Subsequent isolation and acidification of the aqueous layer re-precipitates the benzoic acid which can then be isolated by vacuum filtration. The pH of solutions of carboxylic acids is therefore less than 70. They are more stable than benzoic acid more soluble in water and work best at pH levels between 2 and 44.
 Source: home.miracosta.edu
Source: home.miracosta.edu
It is the simplest aromatic carboxylic acidThe name is derived from gum benzoin which was for a long time its only sourceBenzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food. However the solubility of this compound in water increases when the temperature is increased as is the case with most compounds. Organic compounds soluble in organic solvents but not in water. It is the simplest aromatic carboxylic acidThe name is derived from gum benzoin which was for a long time its only sourceBenzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites.
 Source: degruyter.com
Source: degruyter.com
What would be the maximum theoretical percent recovery from crystallization of 500 g of solid X from 100 ml water. A stronger base sodium hydroxide is required to react with the less acidic 2-naphthol. Aspirin is only slightly soluble in water so if you add it to the reaction mixture the product would rather clump together than be in the water phase. The Vacuum Method No. Journal of Applied Spectroscopy reports on key applications of spectroscopy in physics chemistry material science medicine biology ecology and spectral instrument-industry.
 Source: sciencedirect.com
Source: sciencedirect.com
They are more stable than benzoic acid more soluble in water and work best at pH levels between 2 and 44. A stronger base sodium hydroxide is required to react with the less acidic 2-naphthol. The solubility of carboxylic acids and amines is so characteristic that solubility tests alone differentiate these functional groups from all the others in this experiment. We then need to separate the solid from the rest of the unwanted mixture. Benzoic acid will crystallize from the solvent.
 Source: researchgate.net
Source: researchgate.net
Colours Synthetic Water-soluble Semi-quantitative. As an example methane is soluble in ethanol diethyl ether benzene. It is the simplest aromatic carboxylic acidThe name is derived from gum benzoin which was for a long time its only sourceBenzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. But benzoic acid is not soluble in water. The residual impurities in the organic layer will then be analyzed and identified using gas chromatography.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title benzoic acid water soluble by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.