Benzoic acid soluble in water
Home » chemistry » Benzoic acid soluble in waterBenzoic acid soluble in water
Benzoic Acid Soluble In Water. An increasing number of papers presented here focus on the theory of lasers as well as on the tremendous potential for the practical applications of lasers in numerous fields and industries. It occurs naturally in prunes cinnamon and cloves. PH level potassium sorbate and sodium benzoate. Laboratory irradiation of o-chlorobenzoic acid in deoxygenated water using a medium pressure mercury lamp produced o-hyroxybenzoic acid and benzoic acid1.
 Oneclass Look Up The Solubility Of Benzoic Acid In Hot Water According To The Published Solubility From oneclass.com
Oneclass Look Up The Solubility Of Benzoic Acid In Hot Water According To The Published Solubility From oneclass.com
4-Aminobenzoic acid also known as para-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the para position is an organic compound with the formula H 2 NC 6 H 4 CO 2 H. It is slightly soluble in water. Once the solution is acidic place the flask in an ice-water bath for 5 minutes to complete the crystallization. The melting points of Acetanilide Unknown A and Acetanilide plus Unknown A were the following. Note that 14. Subsequent isolation and acidification of the aqueous layer re-precipitates the benzoic acid which can then be isolated by vacuum filtration.
Benzoic Acid was recrystallized with a 41 recovery using 95 ethanol and water as the mixed-solvent.
Benzoic acid will crystallize from the solvent. Once the solution is acidic place the flask in an ice-water bath for 5 minutes to complete the crystallization. The free acid form is poorly soluble in water and the sodium salt sodium benzoate is often used because of its greater solubility Fig. This causes the solid to begin to form or precipitate. Carboxylic acid unknown options Part 1. It is the simplest aromatic carboxylic acidThe name is derived from gum benzoin which was for a long time its only sourceBenzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites.
 Source: degruyter.com
Source: degruyter.com
The Vacuum Method No. Both of compounds are carboxylic acids. Subsequent isolation and acidification of the aqueous layer re-precipitates the benzoic acid which can then be isolated by vacuum filtration. Collect the crystals of benzoic acid by vacuum filtration using a small Hirsch funnel. Dry Matter in Foodstuffs.
 Source: researchgate.net
Source: researchgate.net
The solubility of carboxylic acids and amines is so characteristic that solubility tests alone differentiate these functional groups from all the others in this experiment. This causes the solid to begin to form or precipitate. It is the simplest aromatic carboxylic acidThe name is derived from gum benzoin which was for a long time its only sourceBenzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. 4-Aminobenzoic acid also known as para-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the para position is an organic compound with the formula H 2 NC 6 H 4 CO 2 H. Once the solution is acidic place the flask in an ice-water bath for 5 minutes to complete the crystallization.
 Source: sciencedirect.com
Source: sciencedirect.com
Introducing the two sodium salts to hydrochloric acid will effectively replace the original proton benzoic acid and 2-naphthol lost. In a separatory funnel containing ether and water it would reside in the ether layer. The melting points of Acetanilide Unknown A and Acetanilide plus Unknown A were the following. What would be the maximum theoretical percent recovery from crystallization of 500 g of solid X from 100 ml water. Salts of benzoic acid are used as food.
 Source: ochemonline.wordpress.com
Source: ochemonline.wordpress.com
14-dimethoxybenzene mp 57 naphthalene mp 82 dibenzalacetone mp 110-111 or benzoin 137. CHEM 2423 Recrystallization of Benzoic Acid Dr. Collect the resulting crystals by vacuum filtration using a water aspirator and the apparatus shown in your laboratory textbook. PH level potassium sorbate and sodium benzoate. 14-dimethoxybenzene mp 57 naphthalene mp 82 dibenzalacetone mp 110-111 or benzoin 137.
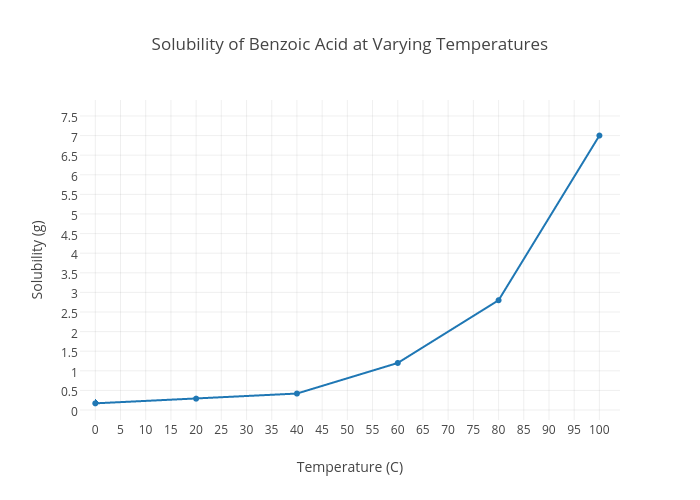 Source: chart-studio.plotly.com
Source: chart-studio.plotly.com
In a chilled environment both compounds will not be soluble in water because the solubility of benzoic acid. Colours Synthetic Water-soluble Semi-quantitative. The other two compounds remain neutral still dissolved in the diethyl ether. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. Note that 14.
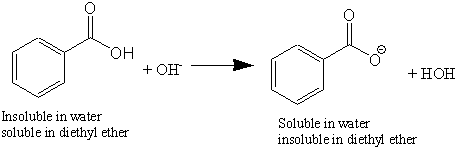 Source: home.miracosta.edu
Source: home.miracosta.edu
Benzoic acid is not very soluble in water. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with. Some organic compounds are soluble only in organic solvents. Aspirin is only slightly soluble in water so if you add it to the reaction mixture the product would rather clump together than be in the water phase. 4-chloroaniline mp 68-71 or ethyl 4-aminobenzoate mp 90 Neutral options same choices for both Part 1 and Part 2.

Determination According to SBR in Meat and Meat Products No. When a basic solution is added to the funnel the acidic compound is deprotonated and becomes an ionic salt. Subsequent isolation and acidification of the aqueous layer re-precipitates the benzoic acid which can then be isolated by vacuum filtration. As we will learn when we study acid-base chemistry in a later chapter carboxylic acids such as benzoic acid are relatively weak acids and thus exist mostly in the acidic protonated form when added to pure water. In a separatory funnel containing ether and water it would reside in the ether layer.
 Source: oneclass.com
Source: oneclass.com
Both of compounds are carboxylic acids. According to Battey et al. They are more stable than benzoic acid more soluble in water and work best at pH levels between 2 and 44. PH level potassium sorbate and sodium benzoate. Aspirin is only slightly soluble in water so if you add it to the reaction mixture the product would rather clump together than be in the water phase.
 Source: researchgate.net
Source: researchgate.net
Benzoic acid was also recrystallized with a 79 recovery using water as the solvent. Collect the resulting crystals by vacuum filtration using a water aspirator and the apparatus shown in your laboratory textbook. The remaining two-component mixture in the ether layer can then be separated and 2-naphthol is then extracted from the remaining mixture. Colours Synthetic Water-soluble Semi-quantitative. 14-dimethoxybenzene mp 57 naphthalene mp 82 dibenzalacetone mp 110-111 or benzoin 137.
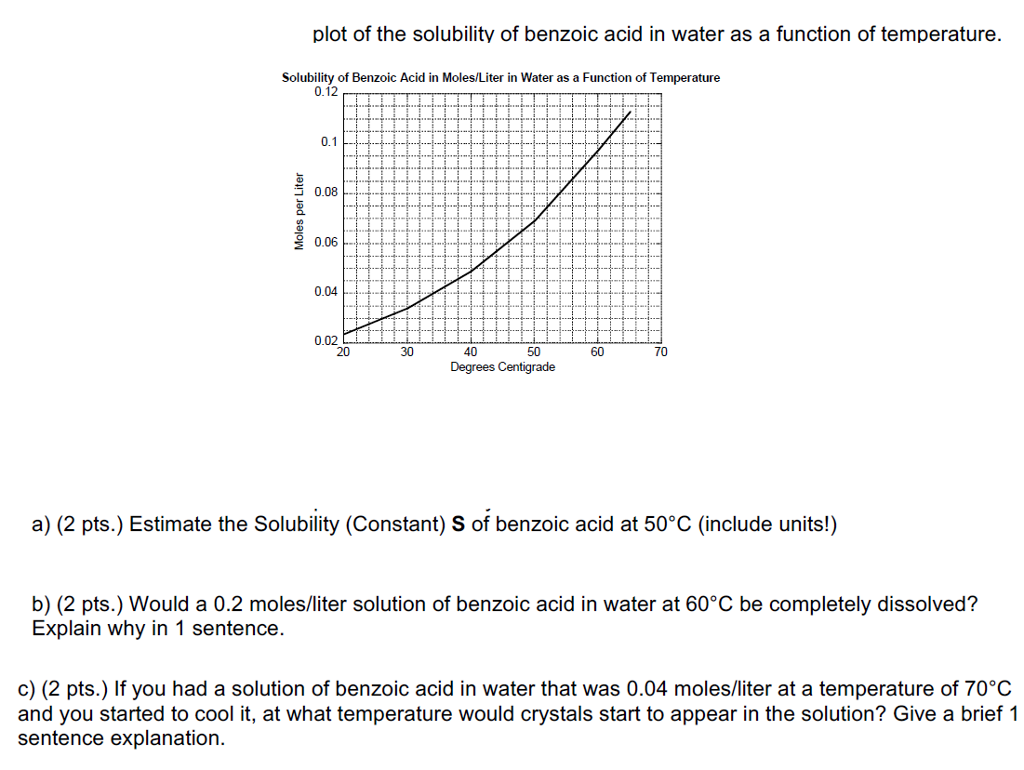 Source: chegg.com
Source: chegg.com
Subsequent isolation and acidification of the aqueous layer re-precipitates the benzoic acid which can then be isolated by vacuum filtration. Introducing the two sodium salts to hydrochloric acid will effectively replace the original proton benzoic acid and 2-naphthol lost. What would be the maximum theoretical percent recovery from crystallization of 500 g of solid X from 100 ml water. Subsequent isolation and acidification of the aqueous layer re-precipitates the benzoic acid which can then be isolated by vacuum filtration. You can derive benzoic acid chemical structure C6H5COOH from benzene by uniting of the water insoluble benzene molecule with a carboxylic acid group -COOH.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title benzoic acid soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.