Acetylsalicylic soluble in water
Home » chemistry » Acetylsalicylic soluble in waterAcetylsalicylic soluble in water
Acetylsalicylic Soluble In Water. The pKa of ketorolac is 35. Other over the counter multivitamins should be avoided unless prescribed by your kidney doctor. Acetylsalicylic acid Aspirin is an organic acid. Be removed from aspirin which is less polar and interacts with the ethanol portion of the mixture.
 Acetylsalicylic Acid Synthesis And Purity Test From ukessays.com
Acetylsalicylic Acid Synthesis And Purity Test From ukessays.com
The juice within the core is extracted by a hot water process and then further treated with a combination of enzymes and high temperature conditions to break down flavorless inulin starch into soluble sugars. Weigh out salicylic acid 30 g 01 g in a clean dry 100 mL conical flask. Check out these. Therefore it is soluble in an organic solvent diethyl ether but will react with a basic reagent B such as sodium hydroxide or sodium bicarbonate to produce the conjugate base of the acid. Sucrose as a polymer of glucose and fructose is a white crystal with a sweet taste that is used as a sweetener in world cuisine. International Journal of.
The apparatus used consists of a Buckner.
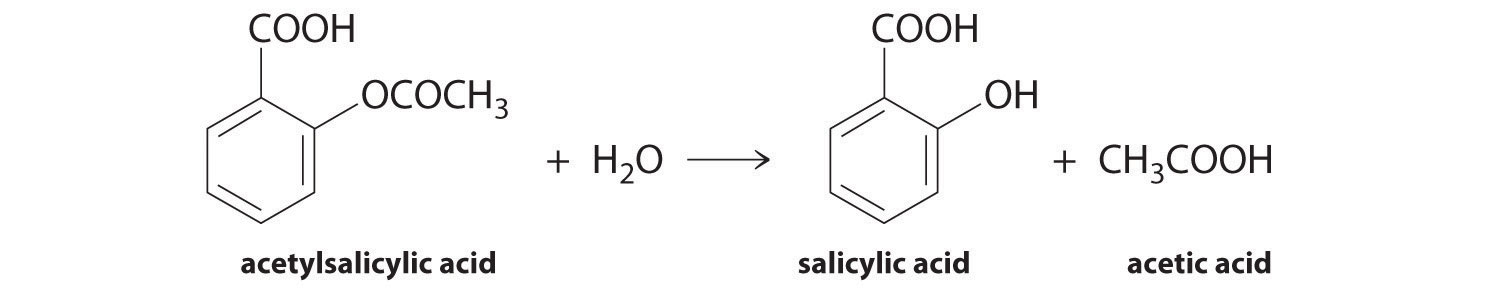
It has a complex structure and a good amount of. This will cause the precipitation of the acetylsalicylic acid and will react with any remaining acetic anhydride. Also known as Aspirin acetylsalicylic acid ASA is a commonly used drug for the treatment of pain and fever due to various causes. Good idea but the science doesnt support it. Generally known as aspirin it is a crystalline solid that is synthesized as a medication to treat pain and inflammation. Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease pericarditis and rheumatic fever.
 Source: ibalchemy.com
Source: ibalchemy.com
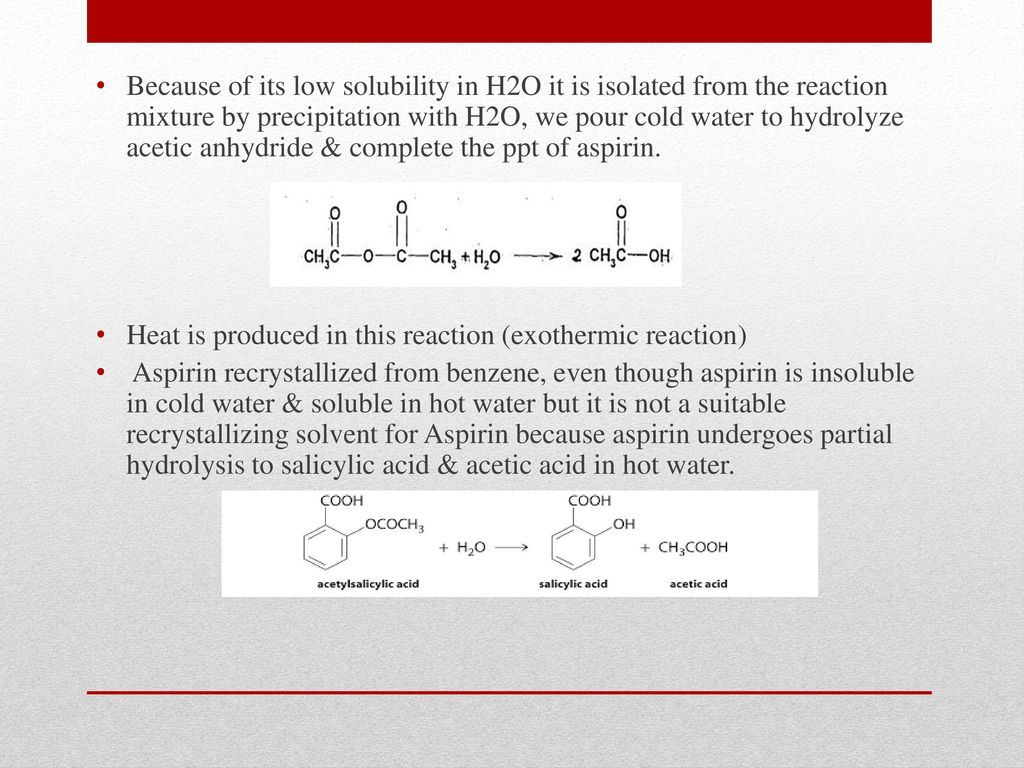
The genesis of the idea cam from the notion that copper is a great fungicide but the copper in pennies is not soluble in water. 7- Acetylsalicylic acid. Dry an Erlenmeyer flask and add 3 grams of salicylic acid to it. So if there is Pb 2 and then chloride Cl-ions in significant. Acetylsalicylic acid is not very soluble in cold water 025 g per 100 mL and consequently it can be isolated by diluting the reaction mixture with water and filtering off the solid product.
 Source: researchgate.net
Source: researchgate.net
Pre-admission acetylsalicylic acid therapy and impact on in-hospital outcome in COVID-19 patients. The juice within the core is extracted by a hot water process and then further treated with a combination of enzymes and high temperature conditions to break down flavorless inulin starch into soluble sugars. The pKa of ketorolac is 35. Other over the counter multivitamins should be avoided unless prescribed by your kidney doctor. Therefore it is soluble in an organic solvent diethyl ether but will react with a basic reagent B such as sodium hydroxide or sodium bicarbonate to produce the conjugate base of the acid.
 Source: sciencemadness.org
Source: sciencemadness.org
Aspirin is a drug that is usually used to relieve minor aches and pain and other medical uses such as anti-inflammatory medication. The objective is to isolate pure acetylsalicylic acid. The organic liquids that are also present in the developing solvent serve as the mobile phase. The genesis of the idea cam from the notion that copper is a great fungicide but the copper in pennies is not soluble in water. To ensure that there is no longer Pb 2 is useful an indirect assay.
 Source: slideplayer.com
Source: slideplayer.com
The acetic acid is very soluble in water and can. Aspirin Titration with base 6. Aspirin given shortly after a heart attack decreases the risk of death. 135 gmL Wikidata Q18216. Sulfuric acid is unusual in that it is a strong acid when it donates its first proton Equation ref438 but a weak acid when it donates its second proton Equation ref439 as indicated by the single and double arrows.

Check out these. Be removed from aspirin which is less polar and interacts with the ethanol portion of the mixture. 14 gmL Alfa Aesar A12488. Generally known as aspirin it is a crystalline solid that is synthesized as a medication to treat pain and inflammation. In a nonelectrolyte system sucrose is utilized.
Source: quora.com
Note that an aspirin tablet contains other compounds in addition to aspirin. A hot water ethanol mixture about 20 mL hot solvent of waterethanol per gram crude aspirin is used to further purify aspirin by removing acetic acid. Although acetic acid is very soluble in water almost all of the acetic acid in solution exists in the form of neutral molecules less than 1 dissociates. Aspirin acetylsalicylic acid is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. Sulfuric acid is unusual in that it is a strong acid when it donates its first proton Equation ref438 but a weak acid when it donates its second proton Equation ref439 as indicated by the single and double arrows.
 Source: ukessays.com
Source: ukessays.com
Add more distilled water mixing thoroughly with each addition. Most Cited Previous 3 Years International standardization of diagnostic criteria for microvascular angina. Also known as Aspirin acetylsalicylic acid ASA is a commonly used drug for the treatment of pain and fever due to various causes. Synthesize acetylsalicylic acid a. Aspirin is a drug that is usually used to relieve minor aches and pain and other medical uses such as anti-inflammatory medication.
 Source: youtube.com
Source: youtube.com
The solid aspirin will be collected using vacuum filtration. The chemist waited for a long time and afterwards wrote the observation The physicist and the biologist are soluble in ocean water Want to improve your science skills. The organic liquids that are also present in the developing solvent serve as the mobile phase. Ketorolac tromethamine may exist in three crystal forms. A purified product is obtained after.
 Source: youtube.com
Source: youtube.com
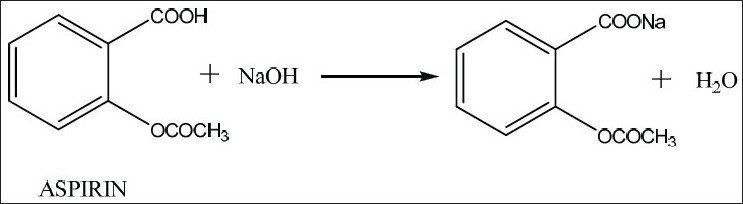
So if there is Pb 2 and then chloride Cl-ions in significant. Any other reaction ingredients that are soluble this includes acetic acid phosphoric acid. Acetylsalicylic acid has both anti-inflammatory and antipyretic effects. Water a component of the developing solvent forms hydrogen bonds with the fibers of the paper and serves as the stationary phase. Synthesis of AspirinAcetylsalicylic acid C 9 H 8 O 4.
 Source: researchgate.net
Source: researchgate.net
Add more distilled water mixing thoroughly with each addition. To the warm solution add 20 drops of cold water drop wise. Aspirin Titration with base 6. Your solution will be cloudy due to insoluble components of the tablet. The solid aspirin will be collected using vacuum filtration.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title acetylsalicylic soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.